
The Value of Transperineal Three-Dimensional Pelvic Floor Ultrasound in the Observation of Pelvic Support in Primipara Women
*Corresponding Author(s):
Qin LiMedical Imaging Department, Shanghai Jiahui International Hospital, 689 Guiping Road, Shanghai, 200233, China
Tel:+86 18930173503,
Email:liqinlw@sina.com
Abstract
Background: The pelvic floor support is weakened during pregnancy and delivery, probably leading to the pelvic floor dysfunction later on in life. An early assessment of the weakening of pelvic support can aid to prevent from developing Pelvic Floor Dysfunction (PFD).
Objective: To explore the ultrasonographic appearance of pelvic support in postpartum compensatory status by Three-Dimensional (3D) transperineal ultrasound.
Methods: This prospective study included 150 primiparas. 3D transperineal ultrasounds were acquired during the first trimester and at 1-month postpartum. Three images were acquired; at rest, during the contraction maneuver, and while performing the Valsalva maneuvers. The Levator Hiatus (LH) size, the angle from the Perineal Body to the LH (PBLH), and the angle from the LH to the Pubic Symphysis (LHPS) were measured on all 3 images. The difference between prenatal and postnatal pelvic measurements were compared.
Results: Compared with the prepartum women, the LH, LHPS, and PBLH were larger in postpartum (P<0.05), either at rest or during the Valsalva maneuver. However, no difference of LHPS was noted in the contraction maneuver.
Conclusion: 3D transperineal ultrasound can reliably assess the change of pelvic support in postpartum, as shown by the increase in the LH, PBLH, and LHPS measurements postpartum at rest and during the Valsalva maneuver. The LH, PBLH, and LHPS ultrasound measurements could be used to observe the function of pelvic support postpartum.
Keywords
Levator ani; Levator hiatus; Levator plate; Pelvic support; Perineal body; Ultrasound
Introduction
The pelvic floor consists of a group of muscles, ligaments, and connective tissue that form a sling or hammock across the bottom of the pelvis. These muscles and other structures support the pelvic organs, including the bladder, uterus, and rectum, and help to control the release of urine, feces, and gas. PFD refers to a condition where the muscles and tissues of the pelvic floor are not functioning properly. PFD affects about 30% of the adult female population [1,2]. Pregnancy and delivery are 2 important risk factors for developing PFD [3-5]. During pregnancy, a series of changes occur in the pelvic floor muscle to accommodate the growing fetus and prepare for delivery [6,7]. These changes can weaken the pelvic floor support [8], making the patient more prone to develop PFD and, ultimately, pelvic injuries such as Urinary Incontinence (UI), Anal Incontinence (AI), and Pelvic Organ Prolapse (POP).
Although pelvic floor injuries are highly related to PFD, it is not always accompanied by PFD [9], suggesting that the pelvic support function might still be in a compensatory state without symptoms in the case of minor injury. Therefore, there was a need for early assessing the weakening of pelvic support to prevent from developing PFD. Understanding the pelvic support function in the compensatory state can provide valuable insights for clinicians in the early detection and treatment of PFD and can ultimately reduce the incidence of distressing pelvic injuries later in life, especially in patients at risk of developing pelvic dysfunction. Although the pelvic support has not yet returned to normal, most women do not develop pelvic floor dysfunction, thus, postpartum is a good time to learn the compensatory status of the pelvic support system.
Three-Dimensional (3D) transperineal pelvic floor ultrasound is a widely used imaging technique to assess the pelvic floor muscles [10-12] and surrounding connective tissue [13,14]. Therefore this technique could potentially be used to locate tissue injuries that lead to PFD. In this study, we aimed to learn the ultrasonographic appearance of pelvic support in postpartum compensatory status by Three-Dimensional (3D) transperineal ultrasound, in order to provide the image support for the study in PFD preventation.
Materials And Methods
- Data collection
Primigravid women treated at the gynecological and obstetric department of Shanghai Jiahui International Hospital between December 2019 and June 2023 were recruited in this study. Chinese women aged between 20 to 40 years with a first-trimester ultrasound-confirmed singleton, a Body Mass Index (BMI) between 18 to 23 kg/m2, and plans to deliver at Shanghai Jiahui International Hospital were included in the study. Patients were excluded if they had a history of pelvic floor reconstructive surgery, gynecologic cancer, pelvic radiation, pelvic trauma, pelvic tumor with a diameter of 5.0 cm or more (such as uterine myoma, ovarian tumor, and fallopian tube tumor), or known connective tissue disorders. Women who had preterm birth were also excluded.
This study was approved by the Ethics Committee of Shanghai Jiahui International Hospital, and written informed consent was obtained from all participants.
- Measurement of the pelvic support function
The pelvic support function of the enrolled subjects was assessed during the first trimester (within the 9 weeks of gestation) and after about 1 month (range 35 to 50 days) postpartum. A physical examination was first conducted to assess the severity of pelvic organ prolapse in accordance with the International Continence Society Pelvic Organ Prolapse Quantification (POP-Q) system. The POP-Q system assigns points to specific landmarks along the vaginal wall and uses these points to measure the position of the pelvic organs relative to the hymen. The landmarks are designated as Aa, Ba, C, D, and Ap for the anterior, posterior, cervix, and perineal body, respectively. The examiner then measures the distance between the hymen and the landmark. These measurements are then used to measure the Genital Hiatus (GH), Perineal Body Length (PBL), and Total Vaginal Length (TVL). The severity of the prolapse is determined using a score of 0 to 4 as follows;
- Stage 0: No prolapse (all points are above the hymen)
- Stage 1: Mild prolapse (the most prolapsed point is within 1 cm of the hymen)
- Stage 2: Moderate prolapse (the most prolapsed point is between 1 and 2 cm below the hymen)
- Stage 3: Severe prolapse (the most prolapsed point is more than 2 cm below the hymen)
- Stage 4: Complete prolapse (the most prolapsed point is outside the vaginal opening) [15]
- Assessment of Stress Urinary Incontinence (SUI)
All participants were then asked to complete a questionnaire to evaluate the SUI symptoms. This SUI symptom score ranges from grade I to III, whereby grade 1 indicates urinary incontinence while coughing or sneezing, grade II indicates urinary incontinence while running or picking up objects from the floor, and grade III indicates urinary incontinence while walking or climbing stairs [16].
- 3D-translabial ultrasound examination
All patients underwent a 3D-translabial ultrasound examination using the GE Voluson E8 (GE Medical system, Zipf, Australia) with the RIC 5-9-D 3D volume probe. The ultrasound scans were acquired at rest, under pelvic contraction and during the Valsalva maneuver. All ultrasound scans were acquired in the supine position with an empty bladder and then analyzed offline using the GE 4D Viewing software by an experienced doctor.
A radiologist specialized in ultrasound reviewed the image as follows. The Transverse (TD) and Anteroposterior (AP) diameter of the levator hiatus, as well as the levator hiatus area [17], were measured at rest, during contraction, and during the Valsalva maneuver on the smallest ultrasound plane. The angle from Perineal Body to the LH (PBLH) and the angle from the LH to the Pubic Symphysis (LHPS) were also measured on all 3 images. The PBLH was defined by drawing a line from the midpoint of the puborectalis junction to the lower internal margin of the pubic symphysis and another line from the upper margin of the perineal body to the lower internal margin of the pubic symphysis (Figure 1). The PBLH angle was defined as the angle between these 2 lines. The LHPS angle was defined as the angle between the long axis of the symphysis pubis and the line between the midpoint of the puborectalis junction to the lower internal margin of the pubic symphysis (Figure 1). The differences between all the measurements acquired at rest and those acquired during the contraction and Valsalva maneuvers were calculated.
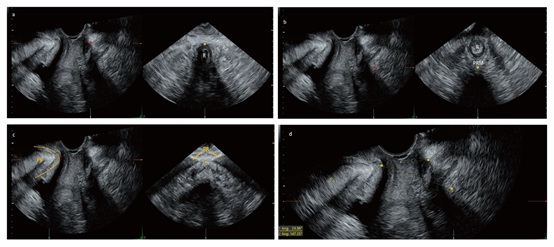 Figure 1: Measurement of the angle from the Perineal Body to the Levator Hiatus (PBLH) and the angle from the Levator Hiatus to the PS (LHPS) in multiplanar mode. Mid-sagittal sections (plane A, left images in panels a, b and c) and coronal sections (plane B, right images in panels a, b, and c) of the pelvic floor in multiplanar mode, in which each anatomic landmark at the sample dot was one-to-one correlated in plane A and plane B. The upper margin of the perineal body in plane A (red dot in panel a) was identified by locating the upper inner margin of the perineal body in plane B (yellow dot in panel a). The midpoint of the PRM in plane A (red dot in panel b) was identified by locating the midpoint of the PRM in plane B (yellow dot in panel b). The PS in plane A (yellow line in panel c) was identified by locating the midpoint of the PR in plane B (yellow dot in panel c). The lower inner margin of the PS (red dot in panel c) was identified accordingly. The PBLH was considered the angle between the line from the midpoint of the PRM to the lower inner margin of the PS and the line from the lower inner margin of the PS to the upper inner margin of the perineal body (panel d). The LHPS was the angle between the line from the midpoint of the PRM to the lower inner margin of the PS and the long axis of the PS (panel d). R: rectum; PRM: puborectalis muscle; PS: pubic symphysis, PR: pubic rami.
Figure 1: Measurement of the angle from the Perineal Body to the Levator Hiatus (PBLH) and the angle from the Levator Hiatus to the PS (LHPS) in multiplanar mode. Mid-sagittal sections (plane A, left images in panels a, b and c) and coronal sections (plane B, right images in panels a, b, and c) of the pelvic floor in multiplanar mode, in which each anatomic landmark at the sample dot was one-to-one correlated in plane A and plane B. The upper margin of the perineal body in plane A (red dot in panel a) was identified by locating the upper inner margin of the perineal body in plane B (yellow dot in panel a). The midpoint of the PRM in plane A (red dot in panel b) was identified by locating the midpoint of the PRM in plane B (yellow dot in panel b). The PS in plane A (yellow line in panel c) was identified by locating the midpoint of the PR in plane B (yellow dot in panel c). The lower inner margin of the PS (red dot in panel c) was identified accordingly. The PBLH was considered the angle between the line from the midpoint of the PRM to the lower inner margin of the PS and the line from the lower inner margin of the PS to the upper inner margin of the perineal body (panel d). The LHPS was the angle between the line from the midpoint of the PRM to the lower inner margin of the PS and the long axis of the PS (panel d). R: rectum; PRM: puborectalis muscle; PS: pubic symphysis, PR: pubic rami.
- Statistical analysis
The data were analyzed using the MedCalc software version 20.1. The means between the three maneuvers were compared using the group t-test, while the means between prenatal and postnatal periods were compared using a paired t-test. A p-value below 0.05 was considered statistically significant.
Results
- Characteristics of the participants
A total of 150 primigravid women met the eligibility criteria and were initially enrolled in the study. Out of these patients, 12 withdrew from the study voluntarily, 4 had a miscarriage, 5 subjects had an induced labor due to fetal anomaly, 1 had a preterm birth at 30 gestational weeks, and 15 missed the postpartum follow-up timing due to the Covid-19 pandemic. Finally, a total of 113 patients were included in the second follow-up phase. Following delivery, none of the participants developed severe POP. Three participant reported persistent mild Stress Urinary Incontinence (SUI) symptoms (grade I) at 1-month postpartum and was excluded from the final statistical analysis of participants with compensatory pelvic function. In addition, fourteen participants reported mild and transient SUI symptoms (grade I) either in late pregnancy or after delivery, but they had no symptoms of SUI after 1-month postpartum and were therefore included in the final statistical analysis. Finally, 110 participants were recruited for this study.
The participants had a mean age of 31 years, an average gestational age at delivery of 39.1 weeks, and a mean birth weight of 3318g. All subjects had epidural anesthesia for pain relief during delivery. Most patients (n=93) had spontaneous vaginal deliveries, 9 had a vacuum-assisted delivery, and 8 had elective cesarean section due to breech. Following delivery, 61 patients had grade I or II vaginal tears.
- The ultrasonographic appearance of pelvic support before and after delivery
The ultrasonographic appearance of pelvic support during the first trimester and at 1-month postpartum is summarized in table 1.
|
Parameter |
Prenatal |
Postpartum |
P value |
|
Mean±SD |
Mean±SD |
||
|
TD(cm) |
|
|
|
|
at rest |
3.72±0.34 |
4.06±0.44 |
P=0.0002 |
|
during contraction |
3.56±0.35 |
3.76±0.49 |
P=0.0245 |
|
during Valsalva |
3.89±0.59 |
4.51±0.62 |
P <0.0001 |
|
Δc-r |
-0.16±0.21 |
-0.30±0.34 |
P=0.0348 |
|
Δs-r |
0.17±0.23 |
0.44±0.44 |
P=0.0024 |
|
AP(cm) |
|||
|
at rest |
4.09±0.55 |
4.47±0.61 |
P=0.0006 |
|
during contraction |
3.52±0.45 |
3.75±0.55 |
P=0.0198 |
|
during Valsalva |
4.45±0.81 |
5.12±0.82 |
P <0.0001 |
|
Δc-r |
-0.57±0.34 |
-0.72±0.48 |
P>0.05 |
|
Δs-r |
0.36±0.61 |
0.65±0.57 |
P=0.0038 |
|
HA(cm2) |
|
|
|
|
at rest |
11.03±2.10 |
12.60±2.07 |
P=0.0019 |
|
during contraction |
9.29±1.74 |
10.16±1.63 |
P=0.0208 |
|
during Valsalva |
13.32±4.52 |
16.97±3.72 |
P <0.0001 |
|
Δc-r |
-1.74±1.22 |
-2.44±1.86 |
P=0.0314 |
|
Δs-r |
2.30±3.89 |
4.37±2.80 |
P=0.0049 |
|
PBLH(°) |
|
|
|
|
at rest |
29.21±6.11 |
33.15±7.72 |
P=0.0009 |
|
during contraction |
32.88±7.18 |
36.33±8.00 |
P=0.0013 |
|
during Valsalva |
27.85±6.62 |
30.03±6.68 |
P=0.0355 |
|
Δc-r |
3.68±4.27 |
3.19±6.33 |
P>0.05 |
|
Δs-r |
-1.36±4.14 |
-3.12±5.88 |
P>0.05 |
|
LHPS(°) |
|
|
|
|
at rest |
143.04±6.85 |
147.05±7.80 |
P=0.0122 |
|
during contraction |
135.24±7.84 |
137.04±14.10 |
P>0.05 |
|
during Valsalva |
153.25±8.74 |
158.23±9.19 |
P=0.0008 |
|
Δc-r |
-7.80±6.13 |
-10.01±13.53 |
P>0.05 |
|
Δs-r |
10.21±7.36 |
11.18±7.57 |
P>0.05 |
|
P value |
P <0.0001 |
P <0.0001 |
|
Table 1: Levator hiatus size, the angle from the Perineal Body to the Levator Hiatus (PBLH), and the angle from the Levator Hiatus to the Pubic Symphysis (LHPS) during the first trimester and at 1-month postpartum.
TD: transverse diameter of the levator hiatus (LH); AP: anteroposterior diameter of the LH; HA: levator hiatus area; PBLH: The angle from perineal body to the LH; LHPS: the angle from the LH to the pubic symphysis; Δc-r: difference between the rest and PFM contraction; Δs-r: difference between rest and Valsalva maneuver.
The LH measurements (AP, TD and HA) decreased during contraction and increased during the Valsalva maneuver (P< 0.05) on both the prenatal and postnatal ultrasound. Compared with the prenatal scans, the LH diameters (AP, TD and HA) at rest, during the contraction maneuver, and during the Valsalva maneuver were larger in the postnatal scans (P< 0.05). The variation of all other parameters except for the AP diameter between the rest and the contraction maneuver was also larger postpartum (P< 0.05). An enlargement in PBLH was noted during the contraction maneuver (P< 0.05), while a reduction in PBLH was noted during the Valsalva maneuver (P< 0.05) in both the prenatal and postpartum periods. On the contrary, LHPS was reduced during the contraction maneuver (P< 0.05) but enlarged during the Valsalva maneuver (P< 0.05), in both the prenatal and postpartum periods. Compared with the prenatal scans, both PBLH and LHPS were larger in the postpartum period (P< 0.05) for all three maneuvers, except for the LHPS during the contraction maneuver. In addition, the variations of these two parameters between the rest and the Valsalva and contraction maneuvers did not differ significantly (P>0.05).
Discussion
The pelvic floor muscles and other connective tissues are important in supporting the pelvis [18,19]. PFD is often associated with injury to the pelvic support structure and can result in symptoms like SUI and POP. Pregnancy and delivery are major contributing factors to PFD. The expanding uterus and growing fetus during pregnancy put excessive strain on the pelvic muscles and ligaments, leading to overstretching [1,20]. Hormonal and mechanical changes during pregnancy can also impair the normal function of the pelvic floor muscles and ligaments, which explains why Cesarean Section (CS) is not entirely protective against the development of PFDs [21]. Vaginal delivery is another traumatic event resulting in myogenic, neurogenic, and/or connective tissue damage in the pelvis. Although the pelvic floor muscle function recovers within a year after delivery [6,22], about 5% to 20% of cases develop irreversible PFD, leading to POP or SUI later in life [21]. Since weakening the pelvic support function is a gradual process, pelvic floor injury is not always associated with PFD [9]. Therefore it is important to monitor the pelvic support function during pregnancy and after delivery to minimize the risk of developing PFD later in life. Numerous studies assessed the efficacy of various imaging methods, such as ultrasound or MRI, to monitor pelvic support function are limited. This study aimed to learn the ultrasonographic appearance of pelvic support by 3D transperineal ultrasound to assess pelvic support function through simple anatomical measurements. Our findings indicate that the LH diameter and the PBLH and LHPS angles could be used to assess the pelvic support function on 3D transperineal ultrasound.
Similar to our findings, the LH was also dilated after delivery and related to the development of POP in previous studies [23]. LH enlargement can be viewed as a reduction in the horizontal support of the levator ani due to its anatomical location and mode of action [24], and it indicates an increased risk of Pelvic Floor Dysfunction (PFD) [25]. Therefore in our study, we considered a hyper-enlarged LH as a good index for poor horizontal pelvic support function. This measurement could be used to assess the horizontal pelvic support floor function and to guide treatment.
The pelvic fascia is a network of connective tissue that gets involved in the pelvic support, together with levator ani, to stabilize the pelvic organs, especially under abdominal pressure [18,19]. The perineal body is a fibromuscular pyramidal structure and forms an important portion of the level III [24,26]. In our study, the PBLH angle was used to evaluate the relationship between the perineal body and the levator plate as it reflects the level of tightness and damage of the level III muscles. Our study demonstrated that the connection between level III and pelvic support weakened after birth for both the contraction and Valsalva maneuver, as evidenced by an increased PBLH angle even without PFD. These changes were easy to measure and could provide valuable insight when used in conjunction with the self-control comparison. Our findings suggest that even if there is no obvious evidence of PFD to suspect pelvic support insufficiency during the postnatal period, the PBLH angle on the 3D-transperineal pelvic floor ultrasound could still detect the functional changes of the level III support. Therefore this parameter could be used to monitor the function of level III in pelvic support, even during the functional compensation period. Similar to a previous MRI study, our findings also showed that an enlargement of the PBLH angle indicates a descent in the perineal body [27]. These results suggest that if there is an indication of level III support weakness, the enlarged PBLH angle should be followed with interest.
The levator plate, together with the fascia and ligaments, has an important role in helping keep the pelvic organs in place, preventing the prolapse of the pelvic organs [19]. We also measured the LHPS angle to evaluate the support of the levator plate in the cranial-caudal direction. Our results showed that the weakened support from the levator plate after delivery could be shown by the increase in the LHPS angle on the rest and Valsalva images, indicating a low location of the levator plate. The descent of the levator plate was more prominent in POP [28]. Therefore, an enlarged LHPS angle in postpartum should be monitored as it may indicate a weakening of the levator plate support. However, no significant change in the LHPS angle was noted before and after birth on the contraction ultrasound, suggesting that the contraction function of the pelvic muscles was not altered during delivery.
In a previous similar study, the Levator Plate Descent Angle (LPDA) was measured by transvaginal ultrasound [29]. However, acquiring these images by a transvaginal probe during the Valsalva and contraction maneuvers is difficult. Therefore the transperineal approach provided a simpler, more comprehensive method for the levator plate evaluation. In addition, the 3D ultrasound facilitated the visualization of the relevant anatomical structures, thus ensuring the reliability of the measurements.
Our study has some limitations that have to be acknowledged. The small sample size of the study limits the generalizability of the research findings. Only participants without PFD after birth were analyzed, thus limiting the performance of parameters in postpartum PFD. The lack of long-term follow-up data to validate the findings is another weakness of our study that requires further investigation. In addition, pelvic floor function can be affected by multiple factors, including age, parity, obesity, and delivery mode, which were not assessed in our study. Therefore larger studies are required to evaluate the impact of these factors on the development of PFD postpartum. Finally, this study did not evaluate paravaginal support, which was linked with SUI in previous pelvic floor ultrasound studies [13,14]. Further studies are therefore recommended to evaluate how ultrasound could be used to assess paravaginal support in postpartum.
Conclusion
3D transperineal ultrasound can reliably detect the weakening of pelvic support after delivery, as shown by the increase in the postpartum LH, PBLH, and LHPS measurements at rest and during the Valsalva maneuver. Overall our findings indicate that the LH, PBLH, and LHPS ultrasound measurements could be used to monitor the pelvic support function postpartum.
Acknowledgment
We are grateful to Shanghai Municipal Health Commission for providing financial support to this work (Grant No. 201940119) and to TopEdit (www.topeditsci.com) for the help in editing this manuscript.
Funding
This work was supported by Shanghai Municipal Health Commission (Grant No. 201940119).
Declaration of Competing Interest
The authors have no competing interests relevant to the content of this article.
Ethical Approval
This study did not involve human or animal research.
References
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, et al. (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300: 1311-1316.
- Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, et al. (2007) Symptomatic pelvic organ prolapse: Prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol 109: 1396-1403.
- Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM (2006) Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol 107: 1253-1260.
- Tegerstedt G, Miedel A, Maehle-Schmidt M, Nyrén O, Hammarström M (2006) Obstetric risk factors for symptomatic prolapse: A population-based approach. Am J Obstet Gynecol 19: 75-81.
- Foldspang A, Mommsen S, Djurhuus JC (1999) Prevalent urinary incontinence as a correlate of pregnancy, vaginal childbirth, and obstetric techniques. Am J Public Health 89: 209-212.
- Reimers C, Staer-Jensen J, Siafarikas F, Saltyte-Benth J, Bø K, et al. (2016) Change in pelvic organ support during pregnancy and the first year postpartum: A longitudinal study. BJOG 123: 821-829.
- Van Geelen JM, Doesburg WH, Thomas CM, Martin CB Jr (1981) Urodynamic studies in the normal menstrual cycle: the relationship between hormonal changes during the menstrual cycle and the urethral pressure profile. Am J Obstet Gynecol 141: 384-392.
- DeLancey JO, Kearney R, Chou Q, Speights S, Binno S (2003) The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 101: 46-53.
- Rostaminia G, White D, Hegde A, Quiroz LH, Davila GW, et al. (2013) Levator ani deficiency and pelvic organ prolapse severity. Obstet Gynecol 121: 1017-1024.
- Yan Y, Dou C, Wang X, Xi Y, Hu B, et al. (2017) Combination of tomographic ultrasound imaging and three-dimensional magnetic resonance imaging-based model to diagnose postpartum levator avulsion. Sci Rep 7: 11235.
- Ying T, Li Q, Xu L, Liu F, Hu B (2012) Three-dimensional ultrasound appearance of pelvic floor in nulliparous women and pelvic organ prolapse women. Int J Med Sci 9: 894-900.
- Dietz HP (2017) Pelvic Floor Ultrasound: A Review. Clin Obstet Gynecol 60: 58-81.
- Dou C, Li Q, Ying T, Shui W, Yan Y, et al. (2018) Value of transperineal ultrasound on the observation of paravaginal support. Arch Gynecol Obstet 297: 943-949.
- Shui W, Luo Y, Ying T, Li Q, Dou C, et al. (2020) Assessment of female pelvic floor support to the urethra using 3D transperineal ultrasound. Int Urogynecol J 31: 149-154.
- Haylen BT, Maher CF, Barber MD, Camargo S, Dandolu V, et al. (2016) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J 27: 655-684.
- Schüssler B, Alloussi S (1983) [Ingelman-Sundberg classification of stress incontinence]. Gynakol Rundsch 23: 166-174.
- Dietz HP (2007) Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol 29: 329-334.
- Petros PE, Ulmsten UI (1990) An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl 153: 7-31.
- Norton PA (1993) Pelvic floor disorders: The role of fascia and ligaments. Clin Obstet Gynecol 36: 926-938.
- Routzong MR, Rostaminia G, Moalli PA, Abramowitch SD (2020) Pelvic floor shape variations during pregnancy and after vaginal delivery. Comput Methods Programs Biomed 194: 105516.
- Van Geelen H, Ostergard D, Sand P (2018) A review of the impact of pregnancy and childbirth on pelvic floor function as assessed by objective measurement techniques. Int Urogynecol J 29: 327-338.
- Stær-Jensen J, Siafarikas F, Hilde G, Benth JŠ, Bø K, et al. (2015) Postpartum recovery of levator hiatus and bladder neck mobility in relation to pregnancy. Obstet Gynecol 125: 531-539.
- Sanozidis A, Mikos T, Assimakopoulos E, Athanasiadis A, Tantanassis T, et al. (2018) Changes in levator hiatus dimensions during pregnancy and after delivery in nulliparas: A prospective cohort study using 3D transperineal ultrasound. J Matern Fetal Neonatal Med 31: 1505-1512.
- Ashton-Miller JA, Howard D, DeLancey JO (2001) The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl 207: 1-7.
- Dou C, Li Q, Ying T, Yan Y, Wang X, et al. (2018) Determining "abnormal" levator hiatus distensibility using three-dimensional transperineal ultrasound in Chinese women. Front Med 12: 572-579.
- DeLancey JO (1994) Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol 170: 720-713.
- Wang W, Chen C, Liu Y, Xu L, Wen T, et al. (2022) Comparison of the Perineal Body Between Chinese Women With Pelvic Organ Prolapse and Women With Normal Support by Magnetic Resonance Imaging With 3-Dimensional Reconstruction. Urogynecology (Phila) 28: 778-775.
- Zhang H, Wang Z, Xiao X, Wang J, Zhou B (2022) Dynamic magnetic resonance imaging evaluation before and after operation for pelvic organ prolapse. Abdom Radiol (NY) 47: 848-857.
- Jeong HY, Park DH, Lee JK (2021) Levator plate descent angle in pelvic floor disorders. Tech Coloproctol 25: 1011-1018.
Citation: Li Q, Zhang S, Wang Y, Yu L (2023) The Value of Transperineal Three-Dimensional Pelvic Floor Ultrasound in the Observation of Pelvic Support in Primipara Women. J Reprod Med Gynecol Obstet 8: 147.
Copyright: © 2023 Qin Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

