
Therapeutic Plasma Exchange and COVID-19: A Rapid Review
*Corresponding Author(s):
Sarabjot Singh MakkarLarkin Health System And Larkin University, 700 SW 62nd Avenue, South Miami, FL, 33143, United States
Email:sarabjot.makkar@case.edu
Abstract
Since it was first reported by the China Center for Disease Control and Prevention (China CDC) in December 2019, multiple mechanisms of pathogenesis have been implied in the morbidity and mortality associated with COVID the Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2). Therapeutic Plasma Exchange (TPE), was early used as a treatment alternative for COVID19. After the publication of some initial studies the US Food and Drug Administration, granted Emergency Use Authorization (EUA), and the World Health Organization (WHO) has endorsed it as potentially efficacious therapy for severe critically ill patients. We describe our experience with TPE and examine ongoing trials. We base recommendations on the current available data to provide a guide and recommendations on the treatment and potentially effects of TPE for patients with COVID-19.
Keywords
Coronavirus; Therapeutic plasma exchange; Efficacy; Tolerability; Preliminary evidence
INTRODUCTION
Therapeutic Plasma Exchange (TPE), which was developed by Dr. Peter Dau in the 1970s, warrants the World Health Organization’s consideration for approval as a potentially efficacious therapy in severe and critically ill COVID-19 patients. The 2019 coronavirus pandemic (COVID-19) continues to spread globally with an estimated 27,489,198 confirmed cases and 896,867 fatalities, as reported by the World Health organization by September 8th, 2020. The underlying etiological agent, severe acute respiratory syndrome 2 coronavirus (SARS-CoV-2) is one strain of many coronaviruses responsible for epidemics in the last two decades. After eight months since the first reported case of SARS-CoV-2 in December 31st, 2019, no treatment of proven efficacy has been demonstrated. The underlying cause of the damage caused by COVID-19 is attributed to immune dysregulation referred to as cytokine storm syndrome. However, other mechanisms have been implied as causative of various organ damage such as hyper inflammation, coagulopathy and endothelial dysfunction [1]. The inflammatory cascade is demonstrated by the elevated immune-inflammatory biomarkers such as cystatin C, presepsin, cardiac troponin I, IP 10, ferritin, c-reactive protein (CRP), d-dimer, lactate dehydrogenase and interleukin-6 (IL-6). Several of these markers are used as severity indicators and even as prognostic factors. The use of Therapeutic Plasma Exchange (TPE has been advocated by many as an adjuvant therapy) [2]. The value of TPE was rapidly entertained in patients with COVID-19. The apheresis (from the Greek work “aphaeresis” that means to take away) involved in the process of TPE has the theoretical ability to eliminate some of the pro-inflammatory substances as well as toxin and cellular components of the sick individual. As of September 8th, 2020, eight randomized clinical trials are underway involving TPE use in COVID-19 on clinicaltrials.gov.
UNDERSTANDING THERAPEUTIC PLASMA EXCHANGE
Therapeutic plasma exchange (TPE) is a blood purification technique that is able to remove larger molecular weight toxins. TPE was first used in 1952 to control hyper viscosity in multiple myeloma patients. After that it was then incorporated as a treatment modality in critically ill patients. The indications for TPE are based on the guidelines from the American Society for Apheresis as well as other professional bodies while practical aspects of the delivery do not have any specific recommendations [3]. There are different techniques by which TPE may be performed including centrifugation or filtration. It is often used in critical illnesses as a primary mode of treatment or as an adjuvant therapy. The procedure, summarized in Figure 1, consists on separating the cellular components from the plasma within the collected blood using a semipermeable membrane, technique known as filtration or by spinning the whole blood using centrifugation [4]. Filtration, removes proteins, immunoglobulins, and immune complexes. Because of their size, toxins are often unable to be removed using conventional methods. Once collected, the plasma is then discarded and is consequently replaced with a colloid (e.g. albumin and/or donated plasma) or a combination of crystalloid and colloid solutions. The cellular components are then returned to the recipient. The removal of these toxins is necessary to reduce the ongoing morbidity in critical illnesses to prevent further organ damage by selectively removing large molecular weight substances from the intravascular space or replenishing a deficient factor such as in systemic thrombotic micro angiopathy [5]. In centrifugation, the blood is separated according to their densities with no limitation of the size of molecules. Pharmacological therapies take weeks or months to reduce and eliminate pathological biomarkers within the blood while TPE is able to effectively remove these large molecules providing immediate relief and prevention of further deterioration of the patient’s clinical status.
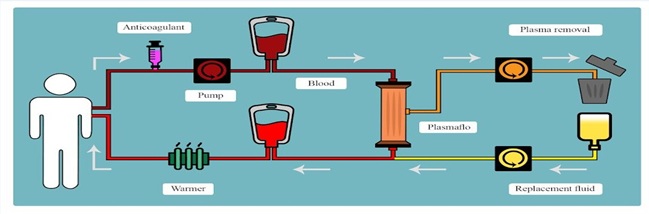 Figure 1: Therapeutic plasma exchange procedure.
Figure 1: Therapeutic plasma exchange procedure.
PROPOSED PROTECTIVE MECHANISMS OF THERAPEUTIC PLASMA EXCHANGE IN COVID-19
TPE is used for a variety of conditions such as Guillain-Barré syndrome, chronic poly neuropathy, thrombotic thrombocytopenic purpura, immune thrombocytopenic purpura, and auto-immune conditions like myasthenia gravis. It acts by removing pathogenic antibodies, complement and clotting factors, immune complexes, cytokines and other macromolecules in the plasma, or albumin-bound small molecules (drugs or toxins) that remain predominantly in the intravascular compartment [6]. The complexity of the inflammatory response in COVID 19 involves multiple of those components. The initial cytokine storm syndrome (CSS) causes widespread inflammation and endothelial damage leading to the activation of the coagulation system and increased vascular permeability [7]. The severity of the cytokine storms has been used as a marker for mortality prediction [8]. Resuscitation using fresh frozen plasma (FFP) has demonstrated efficacy in restoring the integrity of the endothelium microscopically and through assessment of clinical biomarkers [9]. Mortality has also improved in cases of massive hemorrhages when FFP is used concomitantly with TPE [6]. Particularly, the pro-inflammatory manifestations in COVID-19 are attributed to CSS with elevated interleukin-1 (IL-1), interleukin-6 (IL-6), granulocyte-colony stimulating factor (GCSF), tumor necrosis factor (TNF), ferritin, and other immune-inflammatory mediators. The resultant immune injury leads to endothelial damage, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS) [7].
'The recommendations of the American Society for Apheresis (ASFA) for TPE in 2019 did not pertain to Covid19.' [10]. The challenge often remains to determine the moment in time at which the efficacy of TPE may be observed in COVID-19 especially since no specific biomarker or even guidelines exist.
COVID-19 sometimes presents itself as asymptomatic hypoxemia, in some cases with severe degree, also seen as a prognostic indicator [11]. There are two proposed mechanisms explaining the development of lung damage. The first mechanism is the “two activation theory of the endothelium” characterized by two significant molecular mechanisms: 1) release of inflammatory cytokines and 2) activation of platelets with increased presence of unusually large von Willebrand factor multimers (ULVWF) [12]. The second mechanism is the “two-path unifying theory” of hemostasis characterized by microthrombogenesis and vascular micro thrombotic disease (VTD). Both mechanisms are present in COVID-19-associated coagulopathy (CAC) and its associated sequelae. Similarly observed in previous coronavirus epidemics and the H1N1 pandemic, frequently reported outcomes among infected patients are ARDS and MODS. The immune responses in COVID-19 associated ARDS are similar to those present in macrophage activation syndrome (MAS) and secondary hemophagocytic lymphohistiocytosis (sHLH) [13,14]. Given the high mortality risk of ARDS and cytokine storm syndrome among COVID-19 patients, early recognition and control of immune dysregulation is crucial. While many investigational therapies are targeting immune mechanisms, the role of TPE is theoretically effective in removing cytokines and toxins and aborting the cytokine cascade activation.
PRELIMINARY EVIDENCE OF THERAPEUTIC PLASMA EXCHANGE EFFICACY IN COVID-19
First suggested by Keith et al., TPE was proposed as a therapy for critically ill COVID-19 patients [15]. In a case series of 31 COVID-19 patients TPE was beneficial in patients in the intensive care unit (ICU) with either confirmed or imminent ARDS, or pneumonia. TPE administration in COVID-19 patients improved clinical outcomes reducing 28-day mortality (0 vs 35%, p=0.033) and higher extubation rates (73% vs 20%, p=0.018) [16]. In another small case series, three COVID-19 patients with severe illness received TPE after being diagnosed with ARDS. Cytokine storms, characterized by a hyperactive innate immune response, is the release of chemokines, interleukins, interferons as well as other cytokines that pose a risk to the host cells. The reported control of the sequelae of cytokine storms., demonstrated as improved coagulation function and a reduce level of circulating inflammatory markers [17] A case report of a critical COVID-19 patient with respiratory failure and shock identified the benefit of administering TPE in combination with the sequential administration of intravenous immunoglobulin (IVIG) [18].
Several studies have reported the role of TPE among patients with secondary thrombotic microangiopathy (TMA) but not of them specifically targeted COVID-19 patients. A retrospective cohort of 76 patients identified patients with DIC and MODS that had received TPE. The overall survival rates were significantly higher than the controls (82%-vs 20%) promising the use TPE as a rescue therapy [19] Similarly, TPE was an independent contributor to the survival among patients with TMA (hazard ratio=0.845, 95% CI: 0.759-0.940, p=0.002) [20]. Given that COVID-19 patients have higher rates of coagulopathy, the utilization of TPE in COVID-19 has a great potential as a promising treatment.
BENEFIT-RISK PROFILE OF THERAPEUTIC PLASMA EXCHANGE IN COVID-19
For COVID-19 patients with early stages of ARDS, TPE may be beneficial in increasing oxygen saturation levels [21,22]. Complication rates associated with TPE were not of significantly different between 981 elderly and 3728 non-elderly patients that received TPE [23]. A study by Tian et al. on 37 COVID-19 patients receiving TPE near Hubei, China found recovery with no evidence of pulmonary embolism (PE), shock or DIC [24]. Procoagulant depletion in TPE using 5% albumin resuscitation may increase the risk of bleeding [25]. The most common concern with plasmapheresis is hypotension; however, this is commonly rectified by positional change into a Trendelenburg position with raised legs and administration of IV fluids [26]. The anti-coagulants used as part of TPE can also contribute to bleeding. Common complications observed after this procedure include hypotension, hypocalcemia particularly when fresh frozen plasma is used as a replacement fluid, cramping in the muscles, metallic taste in the mouth, and severe immuno-suppression [26]. Allergies may develop to the purification agents used for the TPE tubing as well as to the plasma replacement solutions such as the constituents of 5% albumin solution [26].
ONGOING CLINICAL TRIALS AND FUTURE PROSPECTS
As summarized in Table 1, several ongoing trials have been initiated to determine the outcomes of TPE on COVID-19. Of these trials, two randomized control trials are recruiting patients to compare either the plasma viscosity until 4-day and mortality at day 28 after TPE administration. Many clinical trials are non-randomized and open label aiming to identify overall survival, reduction in cytokines and oxygen needs at different time points.
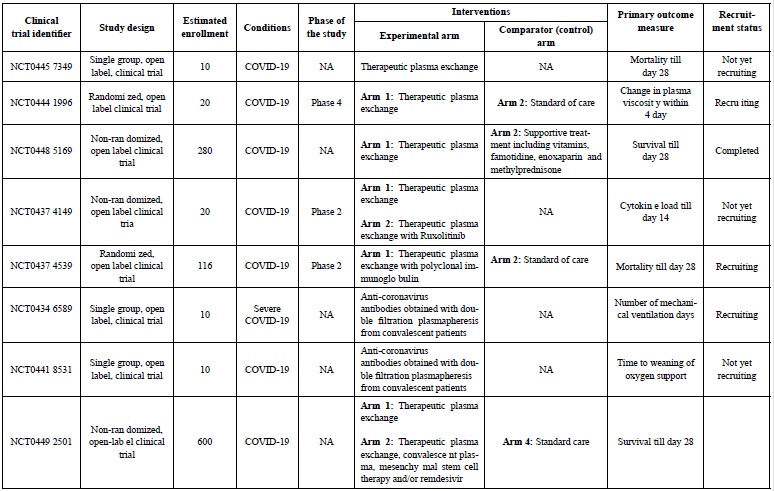 Table 1: Eight ongoing clinical trials involving TPE for COVID-19.
Table 1: Eight ongoing clinical trials involving TPE for COVID-19.
On April 10, 2020, the US Food and Drug Administration (FDA) gave emergency use authorization (EUA) for Terumo’s (OTC: Trumy, formerly Sekisen Ken-onki Corporation) Spectra Optia Apheresis System with Marker Therapeutics’ Depuro D2000 Adsorption Cartridge. Permitted indications included treatment of patients 18 years of age or older with confirmed COVID-19 who were admitted to the ICU with respiratory failure.
RECOMMENDATIONS
Khamis et al. reported positive outcomes with the use of TPE in severe COVID-19 outcomes in a cohort of 31 patients [16]. Given the underlying inflammatory mediators contributing to the morbidity and mortality of COVID-19, TPE might be beneficial, especially in severely ill patients. Importantly, TPE removes toxins and deleterious inflammatory cytokines responsible for cytokine storm syndromes and ARDS, substances that have been repeatedly observed in elevated levels in COVID-19 severely ill patients. Randomized trials are required to better establish the benefits in clinical outcomes COVID-19 patients and to better define specific recommendations for TPE in the COVID-19 pandemic. While no clear recommendations exist, we propose the combination of ABO-matched TPE with transfusions from two different donors that contain effectively diverse titers of SARS-CoV-2 specific neutralizing antibodies (nABs). We also propose that TPE be considered in patients with underlying coagulopathy as TPE may promote the hemostasis needed in these patients and recommend that the international normalized ratio (INR), anti-Xa activity, and activated partial thromboplastin time (aPTT) ought to be monitored in these patients (27).
We recommend the preparation and strict adherence to institutional protocols for the administration of TPE. We call for more trials to better define the adequate timing of administration, number of treatments and volume of plasma to be administered for TPE. It is still to be described and further investigated the administration of TPE in patients receiving prone ventilation.
CONCLUSION
The search for COVID-19 therapies continues with a firm solution still pending due to the formation of new strains and its multi factorial pathogenesis. Among those therapies, preliminary data demonstrates potential benefits of TPE, a well known modality with minimal risks, with potential for use in patients with COVID-19 and ARDS and MODS. However, given the limited experience available, the absence of robust data and the lack of standardization in regards to the timing, volume and number of treatments needed, additional randomized trials on TPE are needed.
ACKNOWLEDGEMENT
No funding was requested for this study. We are thankful to the Larkin Health System for allowing this collaboration to happen.
REFERENCES
- Cao W, Li T (2020) COVID-19: Towards understanding of pathogenesis. Cell Res 30: 367-369.
- Wan S, Xiang Y, Fang W, Zheng Y, Li B, et al. (2020) Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 92: 797-806.
- Puppe B, Kingdon EJ (2014) Membrane and centrifugal therapeutic plasma exchange: Practical difficulties in anticoagulating the extracorporeal circuit. Clin Kidney J 7: 201-205.
- Nguyen TC, Kiss JE, Goldman JR, Carcillo JA (2012) The Role of Plasmapheresis in Critical Illness. Critical Care Clinics 28: 453-468.
- Ahmed S, Kaplan A (2020) Therapeutic Plasma Exchange Using Membrane Plasma Separation. Clin J Am Soc Nephrol 15: 1364-1370.
- Barelli S, Alberio L (2018) The role of plasma transfusion in massive bleeding: Protecting the endothelial glycocalyx? Frontiers in Medicine 5: 91.
- Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: A review. Clinical Immunology 215: 108427.
- Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R (2020) The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology 11: 1446.
- Knaup H, Stahl K, Schmidt BMW, Idowu TO, Busch M, et al. (2018) Early therapeutic plasma exchange in septic shock: A prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care 22: 285.
- Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, et al. (2019) Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher 34:171-354.
- Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN (2020) The pathophysiology of “happy” hypoxemia in COVID-19. Respiratory Research 21: 198.
- Chang JC (2017) Thrombocytopenia in critically ill patients due to vascular microthrombotic disease: Pathogenesis based on “two activation theory of the endothelium.” Vasc Dis Ther 2: 132.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet 395: 1033-1034.
- McGonagle D, Sharif K, O’Regan A, Bridgewood C (2020) The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmunity Reviews 19: 102537.
- Keith P, Day M, Perkins L, Moyer L, Hewitt K, et al. (2020) A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care 24:128.
- Khamis F, Al-Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, et al. (2020) Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis 99: 214-218.
- Zhang L, Zhai H, Ma S, Chen J, Gao Y (2020) Efficacy of therapeutic plasma exchange in severe COVID-19 patients. British Journal of Haematology 26: 181-183.
- Shi H, Zhou C, He P, Huang S, Duan Y, et al. (2020) Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int J Antimicrob Agents 56: 105974.
- Stegmayr BG, Banga R, Berggren L, Norda R, Rydvall A, et al. (2003) Plasma exchange as rescue therapy in multiple organ failure including acute renal failure. Crit Care Med 31: 1730-1736.
- Pene F, Vigneau C, Auburtin M, Moreau D, Zahar JR, et al. (2005) Outcome of severe adult thrombotic microangiopathies in the intensive care unit. Intensive Care Med 31: 71-78.
- Chang JC (2019) Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: Based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb 25: 1076029619887437.
- Tian S, Chang Z, Wang Y, Wu M, Zhang W, et al. (2020) Clinical characteristics and reasons of different duration from onset to release from quarantine for patients with COVID-19 Outside Hubei province, China. Front Med 7: 210.
- Ataca P, Marasuna OA, Ayyildiz E, Bay M, Ilhan O (2014) Therapeutic plasmapheresis in geriatric patients: Favorable results. Transfus Apher Sci 51: 64-67.
- Deng Y, Liu W, Liu K, Fang YY, Shang J, et al. (2020) Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China. Chin Med J (Engl) 133: 1261-1267.
- https://www.mda.org/sites/default/files/publications/Facts_Plasmapheresis_P-206.pdf
- Hodulik KL, Root AG, Ledbetter LS, Onwuemene OA (2019) Effects of therapeutic plasma exchange on anticoagulants in patients receiving therapeutic anticoagulation: A systematic review. Transfusion 59: 1870-1879.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420-422.
Citation: Sarfraz A, Singh-Makkar S, Sarfraz Z, Hathaway III, Paul T, et al. (2020) Therapeutic Plasma Exchange and COVID-19: A Rapid Review. J Clin Immunol Immunother 6: 041.
Copyright: © 2020 Azza Sarfraz, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

