
Thrombosis of Inferior Vena Cava. A New Vision
*Corresponding Author(s):
Gural Romero OscarFlebologia Intervencionista, Fundación Favaloro, Hospital Universitario, Buenos Aires, Argentina
Tel:+54 91136435290,
Email:oagural@gmail.com
Abstract
Keywords
INTRODUCTION
PHYSIOPATHOLOGY
The etiology of the inferior vena cava thrombosis can be very varied, and as we have already mentioned of primary or secondary cause. The first one is related to congenital malformations, alterations during the embryological development of the inferior vena cava, prerenal, renal and post-renal. An alteration in the anastomosis of these segments may favor venous stasis and hypertension responsible for venous thrombosis.
Many authors consider that the clinical profile considered as agenesis of the inferior vena cava in the infrarenal portion are ultimately due to intrauterine thrombosis due to embryological development deficit, which leads to venous stasis and subsequent venous occlusion [1]. It has been reported that 0.3% of the population has some type of congenital alteration of inferior vena cava.
In other publications, 16% to 31% of patients with deep vein thrombosis of the lower limbs and under 50 years old have an abnormality of the inferior vena cava or iliac veins [2-4].
The embryological development of the inferior vena cava is based on the anastomosis of 4 origins or segments:
- Liver segment derived from the vitelline vein
- Adrenal segment derived from right subcardinal vein (hepatic-subcardinal anastomosis)
- Renal segment derived from subcardinal and postub cardinal anastomosis
- Infrarenal segment derived from the right supracardinal vein
Some of the alterations and variants of inferior vena cava malformation are (Figure 1) [5]:
- In the infrarenal segment: Duplication of the inferior vena cava, aplasia of the infrarenal segment, inferior vena cava with retro caval ureter, duplication of inferior vena cava with left retro cavus ureter
- In the renal segment: retro aortic renal vein or circumaortic renal vein
- In the adrenal segment: azygos vein drainage of inferiorvenacava, inferior left venacava followed by hemiazygos vein
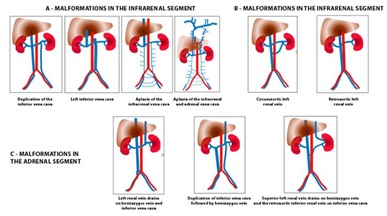 Figure 1: Malformations of inferior vena cava A) More frequent malformations of the infrarenal segment. B) Malformations at the level of the renal segment. C) More frequent malformations of the adrenal segment. Modification of classification of Motta et al. In charge of Gural O, including new variants.
Figure 1: Malformations of inferior vena cava A) More frequent malformations of the infrarenal segment. B) Malformations at the level of the renal segment. C) More frequent malformations of the adrenal segment. Modification of classification of Motta et al. In charge of Gural O, including new variants.
Secondary inferior vena cava venous thrombosis is mainly due to extrinsic compressions such as neoplasms, traumatic injuries, etc. Endovascular intervention such as implantation of non-removable vena cava catheters and filters favors thrombosis. The associated pro-thrombotic factors are prolonged immobilization, pregnancy, obesity, gynecological, urological or traumatological surgeries, coagulation abnormality and paraneoplastic states.
The incidence of neonatal thrombosis is estimated at 2.4 per 1,000 patients in an intensive care unit, 89% is due to the presence of a central venous catheter, and 29% due to sepsis secondary to a catheter. Many of the patients who in adulthood present inferior vena cava thrombosis refer to the history of venous cannulation implants in their childhood, such as the implantation of central catheters for prolonged antibiotic therapy or parenteral nutrition [6].
Compressive venous syndromes of the abdomen such as May-Thurner syndrome are factors that contribute to iliofemoral venous thrombosis, which in certain situations may extend to inferior vena cava by less than 6%, associated with comorbidities already described. The reason for the low progression of the iliac thrombosis to the inferiorvena cava is due to the venous protective effect generated by the septa present in the sequential iliac vein, which acts as a lower vein filter [7,8].
Oguzkurt et al., determined that 24% of asymptomatic patients have a 50% occlusion of the left common iliac vein evidenced by CT. Patients with Deep Venous Thrombosis (DVT) of the left leg reported a 74% increase in mean iliac vein occlusion versus asymptomatic controls, and 2/3 of patients with DVT had 70% iliac axis occlusion.
Chung et al., showed a high incidence of iliac occlusion in venography in thrombosis of the right lower limb, especially in women, where the anatomy of the pelvis has greater arterial compression over the iliac vein, so they have a higher risk of Iliac-femoral thrombosis.
Deep Vein Thrombosis (DVT) generates a valvular and venous wall lesion, with the formation of synechiae by partial recanalization, increasing the risk of re-thrombosis and the appearance of occlusive symptoms, venous hypertension and post-thrombosis [9,10].
Different classifications of lesions, occlusions or variants of inferior vena cava malformations have been published, such as the one proposed by Gloviczki et al., in a study group of 66 patients, based on [11]:
- Place of inferior vena cava injury
- Place of ilio-femoral or renal vein injury (Right/Left)
- Inflow
The highest incidence was in cases with aplasia of the inferior vena cava and common and external iliac (40 patients); followed by 14 cases of supra, renal and adrenal vena cava aplasia; 4 cases for infra and adrenal hypoplasia of inferior cava; 4 cases of infrarenal inferior vena cava aplasia, right and left external iliac vein and 2 supra and infrarenal aplasia of inferior vena cava and common left and external iliac veins.
Jason Crowner et al., they proposed an anatomical classification where they divided it into different types of occlusive or stenotic lesions, of one or more segments and included the following vessels, inferior vena cava, common iliac vein, external iliac vein and common femoral vein:
- Type I: stenosis of a single venous segment
- Type II: stenosis of multiple venous segments
- Type III: occlusion of a single venous segment
- Type IV: occlusion of multiple venous segments
In this work, venous stenting was performed, showing better results of primary and secondary permeability in type I and II cases. Type IV showed an occlusion rate of 26.7%, unlike types I with 7.8% and type II with 4.3% [12].
In conclusion, all complications generated in the short and long term are due to venous hypertension generated by occlusion, so all current treatments are based on their resolution.
CLINICAL MANIFESTATION. ACUTE THROMBOSIS AND POST-THROMBOTIC SYNDROME
In acute deep vein thrombosis, the clinical manifestation will depend on the location, if it is partial or total, the degree of compensatory collaterality generated and the associated external factors such as the patient's general condition, neoplasms, immunosuppression or pro-coagulants drug treatments.
Occlusion of the ilio-cavo-femoral axis can be partial or total, uni or bilateral and when it is significant, extensive and with little collateral circulation, it can produce a profile of edema, lower limb pain and functional impotence. In more extreme cases, phlegmasia alba dolens may initially be associated and in even more severe cases, phlegmasia cerulea dolens, characterized by severe edema, cyanosis, compartment syndrome, and severe ischemia associated with high limb risk. These patients require early intervention because it has a high morbidity and mortality.
A group of patients with phlegmasia alba dolens, characterized by thrombosis of an entire axis but with preserved collaterality, improve sign symptomatology with general measures and anticoagulation, although just anticoagulant treatment favors the onset of post-thrombotic syndrome in the medium and long term.
This syndrome is characterized by varicose veins (C2 of CEAP), chronic pain, edema or associated lymphedema (C3 of CEAP), cellulitis, changes in skin coloration such as lipodermatosclerosis (C4 of CEAP) and hypertensive venous ulcer (C6 of CEAP) in more advanced stages.
It has been estimated that ¼ of the extremities with hypertensive venous ulcer have iliac-femoral axis occlusion by 80% or more, secondary to the post-thrombotic condition [13].
Post-thrombotic syndrome has an incidence of 20-50% in patients treated only with anticoagulation, measures taken in most centers, where interventional therapy is not considered or taken into account. An affectation of the ilio-cavo-femoral axis generates 95% of valvular insufficiency, deep venous insufficiency, venous hypertension, lumbar and pelvic pain and lower limb edema that increase with orthostatism, prolonged standing or sitting, after 5 years of the DVT. In addition, signs of venous claudication may occur in 50% of patients, reduction of physical activity due to heaviness or stiffness of the lower extremities, predisposition to recurrent cellulitis of the limb, dermatitis due to stasis, venous ulceration of the leg and involvement of patients' quality of life [14-16].
The venous return of the limb and of the abdominal and intrapelvic organs is affected, giving rise to very varied shunt patterns and a Secondary Pelvic Congestion syndrome (SCP). The presence of non-compensatory or derivative centrifugal collateral circulation can be observed, according to the derivation theory expressed by Dr. Javier Leal Monedero et al., [17].
Derivative venous drainage is evidenced by collateral circulation in the thigh, abdominal wall, gluteal axis and pelvic congestion. The ultrasound and radiological signs that occur in an occlusion of common and external iliac vessels, and vena cava are:
- Flow inversion by internal iliac vein
- Development of collaterality by the posterior cell, presacral plexus, ascending lumbar vessels and paravertebral circulation
- Derivation by azygos and hemiazygos system
A picture of typical venous congestion syndrome with dyspareunia, vulvar and atypical varicose veins and pelvic floor pain may occur. The difference with pure congestive conditions is that collaterality is centripetal and not centrifugal, such differential diagnosis is possible to objectify in venous Doppler-US.
CLINICAL DIAGNOSIS AND COMPLEMENTARY EXAMS
The clinical examination should generally be done with a standing patient, but in severe cases of thrombosis in the dorsal position, since they do not tolerate standing. It is possible to identify an increase in the diameter of the limb with respect to the contralateral which makes us suspect the level of commitment. Temperature decrease, pain and functional impotence are also present. With regard to the coloration of the limb, it is usually of a slight bluish color in cases of presenting permeable and efficient collaterality, characteristic of phlegmasia alba dolens. But when faced with a massive thrombosis with no or little collaterality such as phlegmasia cerulean dolens, where venous return is severely compromised, there is an increase in the signs and symptoms described and the coloration is compromised taking an intense bluish color, with loss or significant decrease of arterial pulses. The examination of peripheral pulses is essential to quantify the compromise.
Blood tests are requested that include complete blood count, blood glucose, uremia, ionogram, hepatogram, coagulogram with prothrombin time, Thrombin, Quick, APTT, platelets, and fibrinogen, since in some cases thrombolytics and oral anticoagulant drugs of last generation are used.
The pelvic, abdominal and transvaginal, and the lower limb venous Doppler-US, can certify the diagnosis. This should include the femoral, popliteal, tibial and twin vessels as well as the inferior vena cava and the renal and iliac veins. The examination may be hindered by the presence of edema, meteorism, obesity and / or pain of the patient. It is possible to identify compressive, post-thrombotic or mixed venous congestion patterns. It is operator dependent and has a significant learning curve.
3D multislice computed angiotomography and nuclear magnetic angioresonance allow solving the limitations of the Doppler-US or resolving cases of diagnostic doubts. These tests require performing with early and late arterial and venous times, trying to identify the site of occlusion, derivative pathways as well as compression syndromes such as May-Thurner or Nutcracker. It also allows to determine anatomical variations of the inferior vena cava, and iliac or renal veins, such as agenesis, double renal vein, retroaortic renal vein, etc., which may be responsible for thrombotic conditions.
In case of deciding on an interventional behavior, the ascending and descending diagnostic phlebography and the selective pelvic phlebography allow to corroborate with certainty the severity of the pathology, the presence of compensatory collaterality pathways generated and to propose an adequate therapy. Pelvic phlebography should be performed in haemodynamic rooms, with neuroleptoanesthesia or general anesthesia and strict patient monitoring.
CONVENTIONAL MEDICAL TREATMENT [14]
Patients with thrombosis of the inferior vena cava, associated or not with iliac and femoral veins, require admission to special care units, since strict monitoring of vital signs, diuresis with placement of bladder catheterization, and parenteral hydration are necessary.
Measures such as limb elevation, elastic or inelastic compression, and phlebotonic and analgesic medications improve the clinical profile but do not resolve venous damage. Elastocompression or elevations of the limb are contraindicated if there is evidence of arterial involvement, since it would increase ischemia with a risk of necrosis.
According to the protocol of the Congestive, Compressive and Thrombosis Syndromes Management Unit of the Favaloro Foundation of Buenos Aires, Argentina, if the patient does not have criteria for eventual interventional therapy, it can be treated as follows:
- If it has preserved renal function: enoxaparin 1 mg / kg every 12 hours + warfarin 5 mg and adjust according to RIN or initiate DOACs according to current protocol (rivaroxaban 15 mg every 12 hours x 3 weeks / apixaban 10 mg every 12 hours x 1 week / dabigatran 150 every 12 hours, the latter after 1 week of enoxaparin)
- If it has moderate-severe renal impairment: Heparin (sodium or calcium) to maintain APTT: 64-80 "and TT> 120" + warfarin 5 mg and adjustment according to RIN
On the other hand, if the patient presents criteria for interventional therapy, it is started with only sodium heparin for continuous transfusion and discontinued 2 hours before the procedure if anesthesia is general and 4 hours before if it is epidural.
Anticoagulant therapy with sodium heparin, Low Molecular Weight Heparin (LMWH) and other new generation anticoagulants, reduces the risk of re-thrombosis and Pulmonary Thromboembolism (PET), but has little dissolutive action of intravenous clots, without correcting the chronic venous hypertension and the resulting long-term tissue damage.
Residual venous occlusion with incomplete recanalization, evidenced by complementary studies, has a higher risk of recurrent DVT.
Case report No. 1: Female 32 years old, multiparous. Contraceptive intake (ACO). Intermittent edema in lower left limb. The 8th month of pregnancy of the last pregnancy evolves with thrombosis of lower limbs, with a picture of Phlegmasia alba dolens. Medical treatment with anticoagulation was chosen for 1 year (Figure 2).
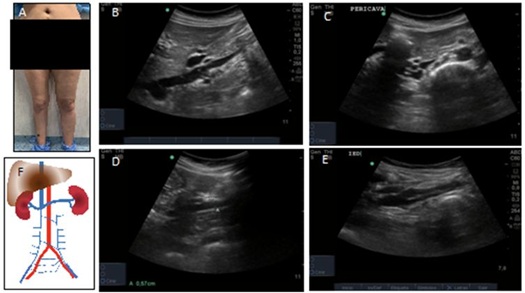
The patient shows signs of pelvic congestion, pelvic pain, dyspareunia, appearance of varicose veins in the abdominal wall and atypical varicose veins, with an inguinal leakage point and right gluteus. The color Doppler-US shows no evidence of infrarenal inferior vena cava until iliac veins with abundant collateral circulation and synechiae in adrenal vena cava and iliac veins. There are also signs of insufficiency of gonadal veins, atypical varicose veins on the front of the thigh in relation to the inguinal leakage point and on the back of the thigh in relation to the right gluteal leak point (Figure 3). Endovascular resolution was proposed, without patient acceptance. Continue to follow up with phlebotonic drugs and elastocompression.
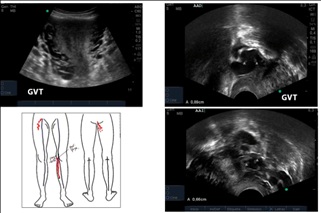
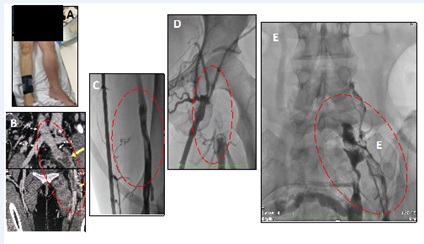
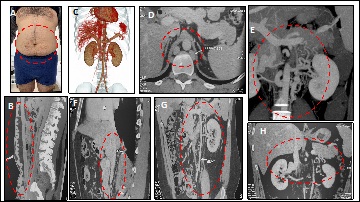
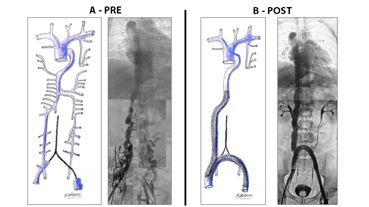
Case report No. 2: Female 46 years old. GAV 1/0/1. History of being a premature mother and underweight, with a bilateral femoral catheter implant for parenteral feeding. She remained asymptomatic, until age 30. She refers chronic pelvic pain, with significant metrorrhagia and severe varicocele. Hysterectomy was proposed as an extreme solution. In the context of evaluation for a living kidney donor, it is verified by venous color Doppler-US and angiotomography, agenesis of infrarenal inferior vena cava and common iliac veins. External iliac veins are observed whose drainage is done by collaterality through left gonadal vein-left renal vein and inferior adrenal vena cava (Figures 4 and 5).

A selective pelvic phlebography was performed where the findings in tomography and Doppler-US were found (Figure 6). The patient refused intervention to evaluate axes restitution using derivative pathways of lumbar, azygos and hemiazygos veins. Continue with anticoagulation, phlebotonic medications and elastocompression.
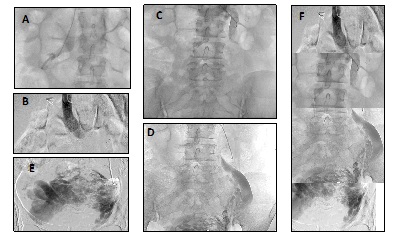
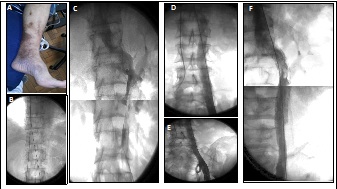
Figure 6: Pelvic selective phlebography. A) Right renal vein that drains into Lower Vena Cava. B, C) Dilated left renal vein, with drainage to the suprarenal inferior Vena Cava. Evidence of reflux by left gonadal vein. D, E) Gonadal vein with severe dilation, severe reflux, associated with severe PCS. F) Reconstruction of photos, showing dilated and insufficient left gonadal vein that drains into dilated left renal vein.
TREATMENT: CONVENTIONAL INTERVENTION VERSUS ENDOVASCULAR
In recent publications, the analysis of the evolution of deep vein thrombosis of abdominal vessels was performed, where two well-defined stages were evidenced. The first until 2000, where 75% of the procedures were performed by open surgery and 25% were endovascular. After that date, 4.1% went through open surgery and the remaining 95.9% was endovascular. The current situation is, endovascular interventions without thrombolysis (53%) or with thrombolysis (33.2%), open surgery (6.8%) and medical treatment (7%) (anticoagulation and elastocompression) [18,19].
In patients with absolute contraindication of anticoagulation, the initial criterion was the inferior vena cava filter implant, a complex situation when the thrombosis compromised the adrenal cava segment. Therefore, the ligation or clipping of the inferior vena cava with high renal thrombosis and general involvement was chosen [20].
Removable and non-removable filters can be used but all have high thrombogenic power. The implant of removable filters is currently recommended, with the possibility of repositioning before 21 days, as an optimal situation [21].
In the following clinical case, thrombosis of inferior vena cava secondary to inferior vena cava filter can be evidenced (Figure 7).
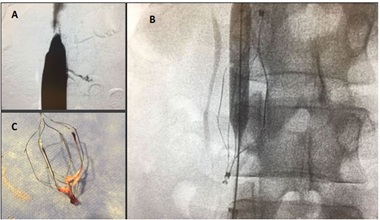
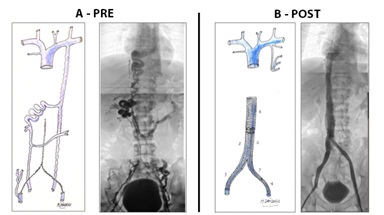
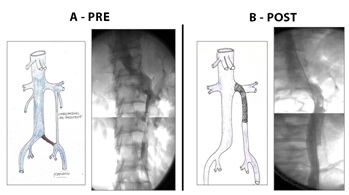
Case report No. 3: Woman, 28 years old, with a history of deep vein thrombosis in the right lower limb, after recent abdominal surgery, with a high risk of bleeding. Color Doppler-US and angiotomography with signs of May-Thurner Syndrome and total thrombosis of the common and external iliac venous axis. Optease vena cava filter is implanted for jugular route. In the 1st week of evolution, clinical progression is evident, with bilateral edema. Inferior vena cava thrombosis is evident at the filter level. Anticoagulant treatment was performed. After 30 days a filter extraction with a loop, thromboaspiration with Penumbra catheter, balloon angioplasty and implant of 2 stents dedicated to vein type Zilver Vena (Cook) is performed. Total restitution of the ilio-cavo axis. Anticoagulant treatment with dicumarinics for 1 year is established.
Among the early complications of the filter implant can be mentioned [21,22]:
- Hematoma or thrombosis in the puncture area, incorrect position of the filter, renal veins, iliac, etc.
- Incomplete opening or not opening. More frequent when the approach is jugular
- Cava drilling
- Migration of proximal filter or right heart
On the other hand among the late complications we can mention:
- Recurrent lung thromboembolism (PET). It is similar with all filters in the current market ranging from 2-5%
- Vena Cava thrombosis. Incidence between 2-22%. Maintain anticoagulation to avoid these thrombosis. 33% exceed the filter proximally
- Flow or cephalic migration. 4-17% of all filters migrate one or two centimeters up or down, without major clinical significance
- Filter breakage
- Postphlebitic syndrome secondary to filter implantation in non-anticoagulated patients
With respect to the different techniques of conventional surgery for the treatment of venous thrombosis, it is possible to mention the femoro-cavo, uni or bilateral iliao-cavo, femoro-femoral or Palma bypass, endarterectomy with patch, venous iliac interposition, etc. Saphenous veins, prostheses, have been used as ducts, where the preparation of distal arteriovenous fistula increases their permeability. Patients should remain prolonged anticoagulated and antiplatelet. Early complications include bridge thrombosis, due to poor inflow or outflow, inadequate conduit, lack of adequate anticoagulation, prosthetic infections, inguinal lymphomas, deep distal venous thrombosis and pulmonary embolism as isolated cases [23,24].
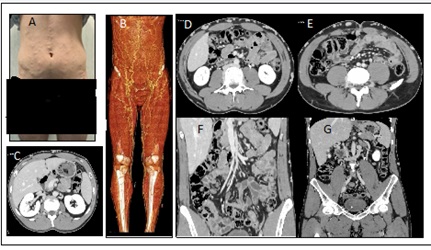
Case report No. 4: Young patient with retroperitoneal tumor, with histology of metastatic testis teratocarcinoma, with infiltration of the right common iliac artery and inferior vena cava. Block tumor resection was performed with vascular reconstruction by iliac-iliac arterial replacement and infrarenal inferior vena cava replacement. Preparation of arteriovenous fistula distal to the replacement of the inferior vena cava, with the aim of increasing the caval venous flow and reducing the risk of venous thrombosis. Primary permeability of 12 years, up to the present [9] (Figure 8).
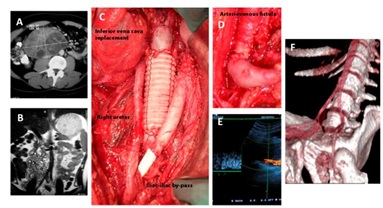
Partial resections and repair with primary closure or patches (autologous or prosthetic), in patients with tumor involvement of the inferior vena cava are very effective and simple to perform.
Case report No. 5: Male, 34 years old with a history of testicular metastatic tumor at the level of the right renal hilum, involvement of the lateral wall of the cava and the right renal artery. Second tumor recurrence. Tumor resection with retroperitoneal lymph node emptying, with safety margins, lateral resection of the inferior vena cava with venous raffia, resection of the renal artery compromised with aortic-renal bypass with inverted saphenous vein (Figure 9).
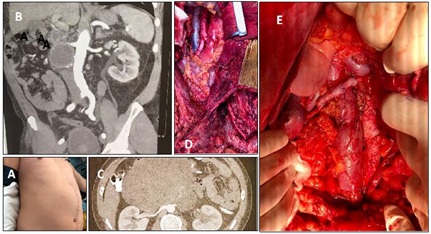
But tumors that involve more than half of the circumference of the vessel wall or with massive intraluminal thrombus require complete resection of the inferior vena cava, to achieve safety margins [25-27].
It is necessary to define previously, the level of cava compromised, for example the adrenal cava is more complex but has greater permeability. The replacement of infrarenal vena cava is simpler but is associated with an increased risk of thrombosis, so that the arteriovenous fistula increases the flow and pressure, decreasing its incidence. Another factor to take into account is whether the vena cava is totally occluded with a satisfactory degree of collaterality through the paralumbar, parietal, azygos, hemizygos, etc., in which case it would have no indication of bypass, only resective surgery. Phlebography plays a very important role in certifying these derivative pathways since it is more difficult to determine compensation with the other diagnostic methods. Other factors to consider are the cancer outcome to be obtained with tumor resection and the general condition of the patient [28,29].
Currently, the management behavior is that of endovascular resolution, in cases of acute thrombosis the combined use of thrombolytics, pharmacomechanical thrombolism and / or thromboaspiration, in the majority of cases associated with balloon angioplasty and stent implantation.
While in chronic thrombosis, angioplasty with a balloon and stent implant is chosen directly. Initially arterial stents were used, such as those of Wallstent Boston, because of their large diameter and length. High rates of primary and secondary permeability have been obtained with this type of stent [30,31].
With the appearance of stents dedicated to veins the results are very satisfactory, with high primary and secondary permeability [32-34]. Anticoagulant treatment decreases the incidence of PTE risk, but has little dissolutive effect of thrombosis, which depends primarily on thrombolysis [35,36].
The combined use of thrombolytics and heparin decreases the incidence of Post-Thrombotic Syndrome (SPT) due to early thrombus lysis and valvular preservation. Systemic fibrinolytic treatment is 4 to 10 times more effective than anticoagulation alone, but its non-localized systemic use may increase bleeding morbidity [37].
Intra-thrombotic selective thrombolism has greater benefits because it allows the impregnation of the fibrinolytic agent and the rapid removal of the thrombus. It has a greater local efficacy with low concentration of systemic drug and lower hemorrhagic complications [38-41].
Among the options for the interventional treatment of deep vein thrombosis we can mention:
- Catheter-directed thrombolysis
Plasminogen Activator (rt-PA)
- Endovascular mechanical thrombectomy
Rheolithic thrombectomy (AngioJet)
Ultrasonic accelerated thrombolysis (EKOS EndoWave)
Rotational thrombectomy (Aspirex catheter, Penumbra)
- Isolated segmental pharmaco-mechanical thrombolysis
Double-balloon catheter (Trellis)
- Endovascular Stent
- Conventional thrombectomy
We can divide the thrombosis according to their time of evolution in acute, subacute and chronic. The approach and behaviors vary, having more favorable results in acute and subacute situations.
Interventional behavior of acute venous thrombosis
According to our protocol they have the possibility of treatment:
- Acute venous thrombosis should be treated within the period considered as optimal, 21 days. The earlier the therapy is performed, the greater the results obtained
- Thrombosis of 2, 3, or 4 levels that compromise the viability of the upper or lower limb and greater risk of developing post-thrombotic syndrome:
- iliac-femoral-popliteal thrombosis
- bilateral cavo-iliac thrombosis
- Flegmasia cerulean dolens or albans independently of the compromised territories
- Patients in good general condition with acceptable life expectancy
Are excluded out of the protocol patients with:
- Thrombosis that exceeds 21 days of thrombosis, since the aspiration thrombolytic therapy has less efficacy. These patients enter into the program of reassessment and eventual programmed endovascular intervention, generally they go to angioplasty and stent implantation
- Patients with isolated infrainguinal, femoral, popliteal, tibial thrombosis that do not compromise the viability of the limb or risk of imminent embolism
- Patients in poor general condition, with renal, heart failure, or multi-organ failure and with low life expectancy
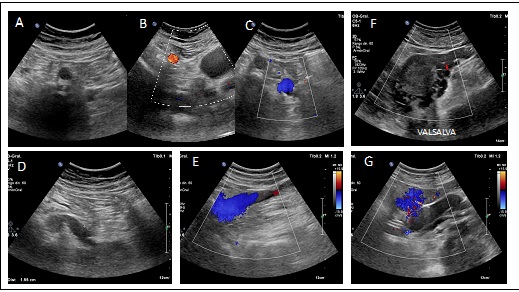
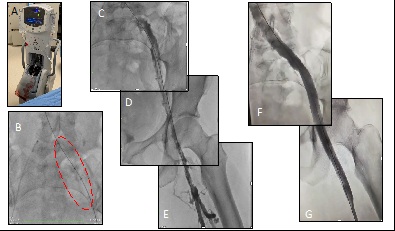
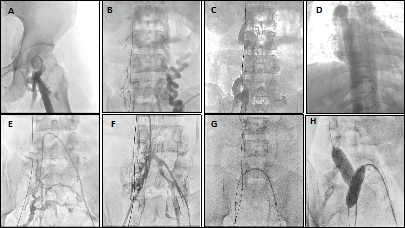
Case report No. 6: A 32-year-old woman had a bladder injury that required raffia during scheduled caesarean section surgery. Reduced mobility, without elastocompression or prophylaxis for DVT. It evolves on the 5th day of hospitalization with significant edema of the lower left limb. By color Doppler-US, partial thrombosis of the inferior vena cava, total thrombosis of the common iliac vein, external iliac, common femoral vein, partial thrombosis of the femoral, popliteal and bilateral twin veins were suspected. AngioTc showed inferior vena cava indemnity, signs of May Thurner with confirmation of thrombosis on the entire left axis. Signs of phlegmasia cerulea dolens, with significant edema, functional impotence, bluish coloration of the limb, absence of pedial and posterior tibial arterial pulses. Anticoagulation is initiated with sodium heparin. Interventional therapy was decided, without a vena cava filter implant, pharmaco-mechanical thrombolism with AngioJet system (Boston Scientific) with rtPA. Two stents dedicated to vein, Zilver Vena (Cook), of 16 x 140 mm and 14 x 90 mm in the iliac and proximal femoral veins were implanted. The patient is discharged at 24h after the procedure with anticoagulation and antiplatelet protocol and the total resolution of the clinical profile (Figures 10 and 11).
In our experience in the Interventional phlebology section of the Favaloro Foundation, we treat cases of deep venous thrombosis in acute, subacute and chronic phase, mostly from a period of 1 to 35 years of evolution. The recanalization of chronic situations where exists fibrosis, stenosis, and very resistant intravascular septae is a challenge. In favorable cases, it is possible to distinguish a filiform passage from iliac, femoral and inferior vena cava, while in others it is impossible to identify such axes, only abundant collaterality.
Technical details for treatment of acute, subacute and chronic thrombosis
- Clinical evaluation, intensive care hospitalization or intermediate therapy
- Monitoring of vital signs, diuresis
- Blood test with evaluation of renal function and coagulogram, thrombin
- -Place of realization. Hybrid hemodynamics room, with an angiograph capable of generating and reconstructing images in 365°
- General anesthesia. Strict monitoring of vital signs
- Bladder catheter to prevent bladder interposition with images and diuresis control
- Triple US-assisted approach with bilateral femoral or popliteal cannulation and right internal jugular vein. The closest to the occlusion site to facilitate angioplasty, with enough space so that the introducer sheath is not interposed
- Approach of the left distal or popliteal femoral vein in the dorsal position according to the site of thrombosis, distal to it and right femoral vein. In situations, the ventral position is chosen to facilitate it.
- Systemic heparinization, ev. Sodium heparin, according to the patient's weight, which is adjusted according to the duration of the procedure
- Placement of 7F introducers
- In other situations we perform ascending phlebography with an US-assisted puncture of superficial veins of the leg or foot, such as the internal saphenous vein, external, or accessory posterior saphenous vein of the leg to perform a mapping of the lesions, with placement of radial introducer of 4F or abocath No. 18 with 3-way key connector. A loop is placed on the leg in the middle third of the leg to facilitate referral to the deep venous system
- The phlebography is done with and without Valsalva maneuvers, and includes the evaluation of superior vena cava, inferior cava, renal, iliac, internal iliac, femoral and popliteal veins. The injection can be manual with 10-20 ml syringe or with injection pump. The use of an injection pump is recommended to facilitate the study, with a flow rate of 6-8ml / s, volume of 10-20ml, Duration 3sec. and pressure 900psi. This allows the entire limb to be evaluated and even iliac vessels with the use of subtraction
- Progress or negotiation through occlusion through femoral vein, iliac vessels, inferior cava, and according to the site of occlusion, with a 0.035 260cm hydrophilic guide with a J-tip and straight. (Type Terumo, Zip wire (Boston Scientific), or Roadrunner (Cook)) and a multipurpose catheter of 5F of 120cm Boston Scientific, or vertebral Terumo of 5F
- In the case of acute thrombosis, a 260mm Amplatz short 0.035 guide is progressed to the pharmaco-mechanical thrombolis process, until healthy inferior vena cava. Intra-thrombus is progressed from distal to proximal with Zelante DVT TM thrombectomy catheter. AngioJet system (Boston Scientific) with rtPA is used in doses of 15 mg / 150 ml of physiological solution, with the Power Pulse TM system. This allows the thrombolytic to be applied directly to the clot allowing pharmacomechanical therapy due to its saturation and softening effect. The thrombus fragmented by the solution jets is absorbed by the catheter by a vacuum effect, Bernoulli effect
- In most cases it is possible to objectify occlusions as a result of joint rupture with guide and vertebral hydrophilic catheters Terumo, multipurpose diagnosis 6F Boston, or right coronary. Preferably by the committed member, or simultaneously from the contralateral side or retrogradely
- We opted for maneuvers performed with 2 operators, one near the introducer that pushes the catheter and the other distal that pushes the guide, which facilitates the procedure
- Continuous testing with contrast with 10ml syringe, to certify the progression of angioplasty, evidence of collaterality, signs of iliac or cavus axes. Venous perforation is frequent, self-delimiting without clinical implication, but requiring continuous evaluation of the general condition and progression of the hematoma
- Once we have overcome the occlusion with a guide and catheter, tested with phlebography evidencing flow in vena cava, we change the first one with a 260 cm Amplatz 0.035 tip short tip
- We change the introducer of the member committed by one of 11 or 12F, and the initial catheter for an 8F guide catheter on Amplaz 0.035 guide of 260cm. We perform ascending phlebography with pump to certify intravascular permanence
- We perform angioplasty with balloons of progressive diameter, 4, 6, 8, 10, 12 mm and of greater length that are available, everything depends on the vessel to be treated
- Testing with phlebography of the progression of balloon angioplasty
- Placement of self-expanding stents dedicated to veins in their preference at the proximal level, with diameters according to the vessel
- We recommend using vein stents:
- 18-20 mm inferior vena cava
- 16-18 mm iliacveins
- 14-16 mm femoral veins
- Among the stents dedicated to veins in our environment and that we have used we can mention Zilver Vena (Cook), Sinus Vena (Optimed), Sinus Oblicus (Optimed), ViciStent Veniti (Boston Scientific) and Venovo (Bard)
- In some circumstances we use arterial stents such as the Wallstent (Boston Scientific) at the distal level, in the external iliac vein and femoral due to budgetary and medical coverage issues
- The stents are ballooned with high pressure 16, 18 or 20 mm Atlas De Bard type balloons, according to the stent diameter
- Simultaneous ascending phlebography in lower limbs in different positions, rotation in 365º to visualize the restitution of the venous axis and need for implantation of another stent or complementary balloon angioplasty
- We do not have IVUS for evaluation, due to budgetary limitations, so the evaluation is optimized in multiple incidents and 3 D reconstruction, angio-3D
- Do not reverse anticoagulation. Extraction of introducers with adequate hemostasis by prolonged selective compression
- Start anticoagulation on the first day with LMWH and Clopidogrel, 75 mg / day. And then go to last generation oral anticoagulants and antiplatelet according to protocol
Post-stenting venous recommendations
- Early wandering and elastocompression- 18 to 24h of hospitalization in general room
- Use of analgesics such as tramadol, codeine. Low back and pelvic pain is common, which can last in more extreme cases to one month
Anti-coagulation and anti-aggregation schemes with:
- Rivaroxaban 15 mg every 12 h + Clopidogrel 75 mg / day for 3 weeks
- Then Rivaroxaban 20 mg / day + Clopidogrel 75 mg / day
- Another scheme with Apixaban 5mg every 12h + Clopidogrel 75 mg / day
- Patient with non-thrombotic iliac lesions (NIVL) ACO at therapeutic range x 3 months + antiplatelet.
- In case of multiple stent implants or with regular inflow or outflow, we recommend keeping the anti-aggregation prolonged
- Follow-up with venous color echodoppler at the 1st week, and first 3 months. Veno-CT at 3 months
In case of bilateral iliac occlusion or vena cava, it is necessary to apply different techniques for restitution of the compromised axes. Different stenting techniques are described. Gloviczki P, Rajú and other authors detailed the techniques [11,42,43]:
- Stent Overlap. Kissing Balloon Technique
- Double Barrel Technique or in pants with Kissing Balloon and Kissing Stent
- Inverted “Y” Fenestration or Piercing
- d-Apposition Technique
Among the tips for venous stenting we can take into account [44]:
- Opt for stents dedicated to veins with diameter and length according to the vessel and these should be positioned about 3cm in healthy vein at the proximal and distal level
- Oversize the vessel 20% to avoid stent migration, and take into account that some stents have a certain degree of retraction during implantation
- Do not leave space between stents, a situation that would favour stenosis or occlusion, and overlap them
- A characteristic sign of an optimal result is the disappearance of collaterality, and absence of contrast stasis
- It is necessary to have an adequate inflow and outflow; otherwise it is the main cause of procedural failure. Including stent implantation at the infrainguinal level to ensure adequate inflow, and in case of femoral vein occlusion, the positioning of the deep femoral vein is chosen
- Use anticoagulation and antiplatelet according to protocol. Many cases of early occlusion are due to non-compliance with these guidelines
We present clinical cases with a diagnosis of inferior vena cava agenesis, where the return of venous return was achieved, using as lumbar veins, azygos and hemiazygos.
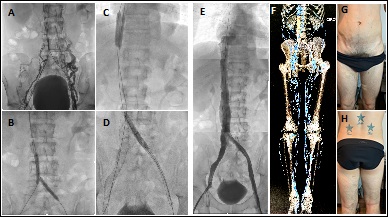
Case report No. 7: Male, 26 years old. From childhood presence of varicose veins in lower limbs, abdomen and thorax. Diagnosis of inferior vena cava agenesis. In adolescence he presented venous claudication, varicocele, and pelvic and lumbar pain. At 25 years of age, he presented an acute picture of edema of the lower limbs, functional impotence and dyspnea. Diagnosis of deep venous thrombosis of bilateral femoral veins was made. There was no evidence of iliac veins or inferior vena cava. Abundant collaterality. Treatment with acenocoumarol, phlebotonic medications and medium compression elastic stockings. Angiotomography and phlebography were performed where there is an absence of inferior vena cava at the infra and adrenal level, absence of iliac vessels, drainage of renal veins in retroaortic horseshoe, right by azygos vein and left by hemiazygos system, and abundant collateral circulation by wall abdominal and pelvic (Figures 12 and 13).
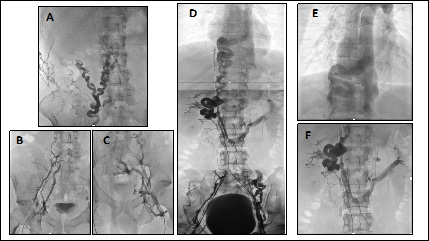
Endovascular treatment was chosen. Combined approach, bilateral femoral in middle third of thigh, and internal jugular vein. It is possible to pass hydrophilic guides through lumbar veins and azygos vein. Angioplasty is performed with progressive diameter balloons, and implant of 1 Sinus Vena 16 x 150mm stent in azygos vein or lumbar collateral; in bilateral lumbar veins with location similar to iliac veins, it was implanted on the left side, 1 Sinus Vena stent 16 x 150mm and 1 Wallstent stent 14 x 95mm and on the right side 3 Wallstent 16 x 95mm stent. In a procedure that lasted 3 hours. He is discharged at 24h with anticoagulation with Rivaroxaban and antiplatelet with Clopidogrel 75mg. During the 1-year follow-up period, the disappearance of venous claudication, pain, collateral circulation significantly and pelvic congestion was evident (Figures 14 and 15).
Case report No. 8: Male, 34 years of age, who suddenly presented lumbar region pain 2 years ago, associated with lower limb edema. A venous color Doppler-US is performed where bilateral deep femoral venous thrombosis is observed. Receive anticoagulant treatment with Acenocoumarol. It evolves with post-thrombotic syndrome, lower limb edema, venous claudication, severe congestion syndrome and hemorrhoids. Abdominal and pelvic venous color Doppler-US and computed angiotomography are performed, showing aplasia of the inferior vena cava, iliac veins, azygos and hemizygous drainage. Retroaortic connected renal veins with azygos vein drainage on the right side and left hemizygos vein (Figure 16).
Triple approach was performed, through the right internal jugular vein, and bilateralby femoral veins in the middle third of the thigh. Aplasia of iliac vessels and inferior vena cavawas evident. To restore the venous return axis, ascending lumbar veins and azygos system were used. It was impossible to connect the left axis to the right side at the abdominal level, only at the distal femoral level, through the left ascending lumbar vein and presacral vein making an endovascular palm system. Angioplasty was performed with progressive diameter balloons. In the azygos and right lumbar veins, 1 Sinus Vena (Optimed) stent of 18 mm x 150 cm was implanted proximally, and on the left side 1 Sinus Vena stent of 14 mm x 150 cm. At the distal level, 2 Wallstent were implanted on the right side and 1 on the left side of 14 mm x 90 cm, in an intervention lasting 2 hours. The patient remains hospitalized 18h and is given anticoagulation with Rivaroxaban 15 mg every 12h and Clopidogrel 75 mg / day (Figures 17 and 18).
Case report No. 9: Male, 30 years. History of deep vein thrombosis in lower limbs. Anticoagulant treatment for 5 years. It evolves with insufficient internal saphenous vein and perforating veins on the inner side of the left leg. Surgery was performed with internal safenectomy and Cigorraga operation of perforating veins. At 2 years later it evolves with new deep infrainguinal left venous thrombosis. Thrombophilia is identified and receives anticoagulant treatment with dicumarinics. It evolves with ocher dermatitis and epifascial varicose veins, which requires new surgery with poor evolution with increased trophic disorders, edema and the appearance of venous claudication. Phlebography was performed with evidence of double inferior cava, where the left side drains into the left renal vein, with duplication with subtotal thrombosis. No evidence of left iliac vein. Angioplasty was performed with an 18 mm x 150 cm Sinus Vena (Optimed) stent implant in the left vena cava to the renal vein. Disappearance of collaterality and significant clinical improvement (Figures 19 and 20).
DISCUSSION
Only 20% of thromboses of the lower limbs with involvement of abdominal and pelvic vessels are restored ad integrum, with anticoagulant treatment only. The rest have long term physical damages giving rise to the post-thrombotic syndrome that appears after the 5th year of evolution. 40% of patients have signs of May-Thurner syndrome according to cadaveric dissection studies, and by angiotomography they go unnoticed, but in situations such as pregnancy, prolonged rest, gynecological or abdominal surgeries, or coagulation disorders can be complicated with deep veinthrombosis of the iliac, femoral and popliteal axes. In some cases (8%), progression of thrombosis to inferior vena cava is evidenced, so it is thought that the Syndrome itself serves as protection for the progression of the condition, due to the partitions generated in the iliac vein.
Secondary venous thrombosis of the inferior vena cava generally presents by compression or tumor invasion, traumatic, or implantation of non-removable vena cava filters. Some patient’s debut with deep venous thrombosis or during a clinical screening, it is possible to show aplasia, hypoplasia or agenesis of the inferior vena cava and iliac vessels. There is a theory that many of these situations occur as a result of a failure in the intrauterine development of the inferior vena cava, resulting from the fusion of the retrohepatic, adrenal, renal and infrarenal segments. In the event of a failure in development, different malformations are generated, being able to occur in all the named segments. The situations of double inferior vena cava, left cava, retroaortic renal veins, circumaortic renal veins, or anomalous renal drainage by azygos and hemiazygos veins, constitute clinical profile of difficulty in the adequate abdominal, pelvic and lower limb drainage. The pathophysiological mechanism is secondary venous hypertension and venous stasis which, given certain risk situations, causes deep venous thrombosis of collateral pathways, iliac vessels and lower limbs. A high incidence of retroaortic renal drainage veins has been evidenced in these situations, such as the clinical cases described, being one of the most frequent malformations [45,46].
In many circumstances it is possible to restore with surgery or endovascular procedures. Currently, the latter opt for excellent results, and high primary and secondary permeability. Traditional anticoagulant treatment in 60% of cases improves the medical history in acute phase, but when we go to a subacute or chronic stage, situations that require endovascular intervention fundamentally appear.
The goal of all endovascular treatments is to avoid or resolve occlusion and therefore venous hypertension. In case of acute thrombosis, a correct evaluation of the patient, inclusion and exclusion criteria, use of pharmacomechanical or mechanical thrombolysis, and where balloon angioplasty and stent implantation is necessary in almost all cases is necessary. In subacute and chronic situations, we depend on multiple factors such as occlusion time, inflow and outflow, length of occlusion and concomitant factors such as thrombophilia, immobility, availability of access to guides, catheters, suitable vein stents, medical coverage covering such practices, etc. The learning curve, such as multidisciplinary management, is necessary to achieve satisfactory results.
CONCLUSION
The deep venous thrombosis of the lower limbs forces us to evaluate the abdomen and pelvis, the iliac axes and the inferior vena cava. The clinical examination should be exhaustive, but complementary examinations such as color Doppler-US, computed angiotomography, angioresonance and phlebography are essential to arrive at the diagnosisand propose the appropriate therapy. They have the limitation of being dependent operators, where we can start from a false negative, bad diagnosis and therefore errors in the treatment with bad results.
The concept that primary inferior vena cava thrombosis is produced by extension of distal thrombosis, forces us to evaluate possible causes of malformations in its development. In most cases it is possible to show aplasias, hypoplasias, duplications, abnormal drainage or retroaortic location of renal veins, responsible for venous stasis and thrombosis.
It is necessary to identify cases that require urgent endovascular therapy and resolve subacute and chronic situations to avoid post-thrombotic syndrome. The development of anticoagulation therapies, pharmaco-mechanical thrombolism, vein stents and pathology knowledge allows us to have excellent short and long term results. It isnecessary to carry out prospective multicenter studies, not only of the use of stents dedicated to vein but of post-stenting measures such as the use of anticoagulants, antiplatelet agents, their duration and the development of appropriate protocols for venous disease.
REFERENCES
- De Blas Zapata A, Pérez-Caballero Macarrón C, Coca Pérez A, Álvarez Rojas E, Vázquez Martínez Jl (2013) Agenesia De La Vena Cava Inferior. Revista Española De Pediatría 69: 132-134.
- Ruggeri M, Tosetto A, Castaman G, Rodeghiero F (2001) Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet 357: 441.
- García-Fuster MJ, Forner MJ, Flor-Lorente B, Soler J, Campos S (2006) [Inferior vena cava malformations and deep venous thrombosis]. Rev Esp Cardiol 59: 171-175.
- Obernosterer A, Aschauer M, Schnedl W, Lipp RW (2002) Anomalies of the inferior vena cava in patients with iliac venous thrombosis. Ann Intern Med 136: 37-41.
- Motta-Ramírez GA, Mundo-Gómez C, Ramírez-Arias JL (2010) La vena cava y sus variantes anatómicas. Rev Mex Angiol 38: 19-29.
- Andrew ME, Monagle P, deVeber G, Chan AK (2001) Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program, Pg no: 358-374.
- Black S, Janicek A, Knuttinen MG (2017) Re-intervention for occluded iliac vein stents. Cardiovasc Diagn Ther 7: 258-266.
- May R, Thurner J (1957) The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 8: 419-427.
- Strandness DE Jr, Langlois Y, Cramer M, Randlett A, Thiele BL (1983) Long-term sequelae of acute venous thrombosis. JAMA 250: 1289-1292.
- Akesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, et al. (1990) Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg 4: 43-48.
- Erben Y, Bjarnason H, Oladottir GL, McBane RD, Gloviczki P (2018) Endovascular recanalization for nonmalignant obstruction of the inferior vena cava. J Vasc Surg Venous Lymphat Disord 6: 173-182.
- Crowner J, Marston W, Almeida J, McLafferty R, Passman M (2014) Classification of anatomic involvement of the iliocaval venous outflow tract and its relationship to outcomes after iliocaval venous stenting. J Vasc Surg Venous Lymphat Disord 2: 241-245.
- Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, et al. (2004) Iliac vein compression In an asymptomatic patient population. J Vasc Surg 39: 937-943.
- Practice Guidelines (2016) Management of Obstruction of the Femoroiliocaval Venous System. American College of Phlebology, Illinois, USA.
- Semba CP, Dake MD (1994) Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology 191: 487-492.
- Semba CP, Razavi MK, Kee S, Sze D, Dake M (2004) Thrombolysis for lower extremity deep venous thrombosis. Techniques in vascular and interventional radiology 7: 68-78.
- Monedero JL, Ezpeleta SZ, Khilnani NM (2016) Treatment options for pelvic congestion syndrome. Phlebolymphology 23: 3.
- La Hei ER, Appleberg M, Roche J (1997) Surgical thrombectomy and stent placement for iliac compression syndrome. Australas Radiol 41: 243-246.
- DeStephano CC, Werner EF, Holly BP, Lessne ML (2014) Diagnosis and management of iliac vein thrombosis in pregnancy resulting from May-Thurner Syndrome. J Perinatol 34: 566-568.
- Ricco JB (2011) Interrupción de la vena cava inferior. EMC - Cirugía General 11: 1-20.
- Tardy B, Mismetti P, Page Y, Décousus H, Da Costa A, et al. (1996) Symptomatic inferior vena cava filter thrombosis: clinical study of 30 consecutive cases. Eur Respir J 9: 2012-2016.
- Teter K, Schrem E, Ranganath N, Adelman M, Berger J, et al. (2019) Presentation and Management of Inferior Vena Cava Thrombosis. Ann Vasc Surg 56: 17-23.
- Norese M, Gural O, Menant A, Padilla E, Scorticati C, et al. (2007) Fístula arteriovenosa distal en el reemplazo de vena cava inferior. Anales de Patología Vascular 1: 71-74.
- Fueglistaler P1, Gurke L, Stierli P, Obeid T, Koella C, et al. (2006) Major vascular resection and prosthetic replacement for retroperitoneal tumors. World J Surg 30: 1344-1349.
- Bower TC, Nagorney DM, Cherry KJ, Toomey BJ, Hallett JW, et al. (2000) Replacement of the inferior vena cava for malignancy: An update. J Vasc Surg 31: 270-281.
- Caldarelli G, Minervini A, Guerra M, Bonari G, Caldarelli C, et al. (2002) Prosthetic replacement of the inferior vena cava and the iliofemoral vein for urologically related malignancies. BJU Int 90: 368-374.
- Yoshidome H, Takeuchi D, Ito H, Kimura F, Shimizu H, et al. (2005) Should the inferior vena cava be reconstruc- ted after resection for malignant tumors? Am J Surg 189: 419-424.
- Quiñónez-Baldrich WJ (1999) Prosthetic Repla- cement of the Inferior Vena Cava. Ann Vasc Surg 13: 449-456.
- Sarkar R, Eilber FR, Gelabert HA, Quiñónez- Baldrich WJ (1998) Prosthetic replacement of the inferior vena cava for malignancy. J Vasc Surg 28: 75-83.
- Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, et al. (2015) Editor's Choice - Management of Chronic Venous Disease Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J VascEndovasc Surg 49: 678-737.
- Brittenden J, Cotton SC, Elders A, Ramsay CR, Norrie J (2014) A Randomized Trial Comparing Treatments for Varicose Veins. N Engl J Med 371: 1218-1227.
- Neglén P, Tackett TP Jr, Raju S (2008) Venous stenting across the inguinal ligament. J Vasc Surg 48: 1255-1261.
- Saha P, Gwozdz AM, El-Sayed T, Karunanithy N, Breen K (2017) Stenting Across the Inguinal Ligament in Post Thrombotic Syndrome Using Nitinol Venous Stents: One-year Patency Outcomes. Eur J Vasc Endovasc Surg 54: 664-668.
- Saha P, Gwozdz A, Hagley D, El-Sayed T, Hunt B, et al. (2017) Patency Rates After Stenting Across the Inguinal Ligament for Treatment of Post-Thrombotic Syndrome Using Nitinol Venous Stents. Journal of Vascular Surgery: Venous and Lymphatic Disorders 5: 148.
- Akesson H, Brudin L, Dahlstrom JA, Eklof B, Ohlin P, et al. (1990) Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg 4: 43-48.
- García-Mónaco R Repermeabilización endovascular en trombosis venosa profunda e interrupción de la vena cava. TROMBOSIS. Tromboembolismo venoso. Fisiopatología, diagnóstico, prevención y tratamiento Pg No: 1-20.
- Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M (2000) Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg 32: 130-137.
- Elsharawy M, Elzayat E (2002 Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg 24: 209-214.
- Semba CP, Razavi MK, Kee S, Sze D, Dake MD (2004) Thrombolysis for lower extremity deep venous thrombosis. Tech Vasc Interv Radiol 7: 68-78.
- Vedantham S, Thorpe P, Cardella FJ, Grassi CJ, Patel NH, et al. (2009) Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 17: 435-448.
- Vedantham S, Padginton C (2005) Percutaneous Options for Acute Deep Vein Thrombosis. Semin Intervent Radiol 22: 195-203.
- García en CIRSE 2013 (2013) Datos finales de PEARL, agosto de 2013.
- Neglén P, Darcey R, Olivier J, Raju S (2010) Bilateral stenting at the iliocaval confluence. J Vasc Surg 51: 1457-1466.
- Rajú S (2015) Catheter Based Interventions In Chronic Venous DiseaseYale School of Medicine, Department of Surgery, Section of Vascular Surgery, Current Management of Venous Disorders.
- O'Donnell TF Jr, Browse NL, Burnand KG, Thomas ML (1977) The socioeconomic effects of an iliofemoral venous thrombosis. J Surg Res 22: 483-488.
- Delis KT, Bountouroglou D, Mansfield AO (2004) Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 239: 118-126.
Citation: Oscar GR, Javier LM, Marcelo D, Oscar M, Luis C, et al. (2019) Thrombosis of Inferior Vena Cava. A New Vision. J Angiol Vasc Surg 4: 032.
Copyright: © 2019 Gural Romero Oscar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

