Transient Monocular Vision Loss in a Patient with Becker Muscular Dystrophy
*Corresponding Author(s):
Deepti BhandareDivision Of Cardiology, Advent Health Sebring, Florida, United States
Tel:+1 863386005,
Email:deepti.bhandare@adventhealth.com
SUMMARY
Becker Muscular Dystrophy (BMD) is a disorder characterized by progressive skeletal muscle failure with eventual cardiac involvement. The diagnosis of BMD is commonly established in early-late adolescence, with adult-onset diagnosis being rare. Cardiomyopathy is the number one cause of death in patients with BMD. Most patients with BMD have asymptomatic cardiac involvement and only up to one third of patients develop dilated cardiomyopathy with symptoms of heart failure. Herein we describe the case of a 38-year-old male with late-onset Becker Muscular Dystrophy with no prior cardiac symptoms presented with sudden transient monocular vision loss and dyspnea. The neurological and ocular exam was within normal. Further evaluation showed patient had developed tachycardia induced cardiomyopathy due atrial flutter causing cardioembolic phenomenon. His symptoms improved with institution of heart failure medications and oral anticoagulation. Involvement of a cardiologist who is integrated into a multidisciplinary care team is recommended as soon as the diagnosis of BMD is made, given the complex decision making involved in managing BMD cardiomyopathy.
BACKGROUND
Becker Muscular Dystrophy (BMD) is an X-linked recessive disorder belonging to a group of disorders known as dystrophinopathies. BMD results from mutations in the dystrophin gene located on chromosome Xp21.1. It is characterized by progressive skeletal muscle failure with eventual cardiac involvement. It affects one in every 18,450 male births. The diagnosis of BMD is commonly established in early-late adolescence, with adult-onset diagnosis being rare. Cardiomyopathy is the number one cause of death in patients with BMD. Most patients with cardiac involvement are asymptomatic and only up to one-third of patients develop dilated cardiomyopathy with symptoms of heart failure.
While cardiac involvement is consistently present in BMD, its severity cannot be predicted by age, degree, duration of skeletal muscle weakness, type of mutation, or presence of cardiac symptoms, which are usually absent. We present this case to demonstrate the unpredictable and insidious course of cardiomyopathic dysfunction in muscular dystrophies as well as to encourage practitioners to immediately implement a comprehensive cardiac assessment and work-up in patients diagnosed with a muscular dystrophy.
CASE PRESENTATION
A 38-year-old male with a past medical history of late-onset Becker Muscular Dystrophy (BMD) presented with transient, intermittent loss of vision in his left eye for three days. Patient was diagnosed with BMD when he was 28 years of age when he developed a wide based gait and lower extremity muscle weakness. Aside from ambulating with a wide-based gait, the patient had no physical restrictions in his daily activities and was able to maintain a walking regimen that consisted of one mile every day. During this presentation, he developed sudden, transient loss of vision three days prior in the left eye which resolved after a few minutes each time. He described it as temporary fogging of the vision in his left eye. This occurred four times over a period of three days with complete resolution of the vision in between these episodes. The patient attributed them to floaters in the eyes and did not give it medical attention. He denied any scalp tenderness and associated headaches.
On the fourth day he developed sudden onset of shortness of breath, NYHA class 3 with orthopnea and pedal edema which prompted the ER visit. On examination the patient had diffuse basilar rales in bilateral lungs, tachycardia with increased respiratory distress and use of accessory muscles. His vision was normal, and he did not have new neurological deficit. Pseudohypertrophy of his calf muscles was also noted. Prior to this presentation, the patient had no history of cardiac symptomatology. Family history was not significant for muscular dystrophies or muscular disorders, but significant for heart disease in the patient’s maternal grandmother.
INVESTIGATIONS
The chest x-ray showed cardiomegaly with bilateral small effusions. The EKG shows atrial flutter with a rapid ventricular heart rate of 140 bpm and intraventricular conduction delay (Figure 1). Labs showed normal serial troponin levels and elevated proBNP levels [4647 pg/ml (normal range is 0-450pg/ml)]. The creatine kinase levels were elevated to five times the normal value, but that was his baseline due to the muscular dystrophy. The WBC count, ESR and C-reactive protein were within normal limits. CT scan of the head and carotid duplex were normal. His visual exam and acuity were within normal limits.
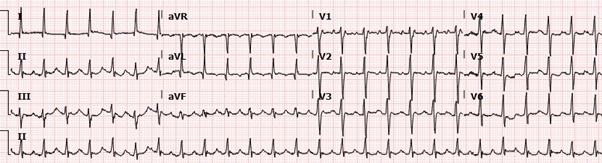
Figure 1: EKG showing atrial flutter with rapid ventricular rate with intraventricular conduction delay.
He was started on a diltiazem drip for rate control and apixaban for oral anticoagulation. An echocardiogram was performed which revealed severe global hypokinesis of the left ventricle with ejection fraction of 20-25% and moderate mitral and tricuspid regurgitation. His heart rate was difficult to control with intravenous calcium channel blockers; hence a transesophageal echo was planned prior to a performing a direct current electrical cardioversion. Transesophageal echocardiogram demonstrated an estimated ejection fraction of 25-30% with moderate left ventricular hypokinesis, mild-moderate tricuspid regurgitation, moderate mitral regurgitation and a thrombus in the left atrial appendage (Figure 2).
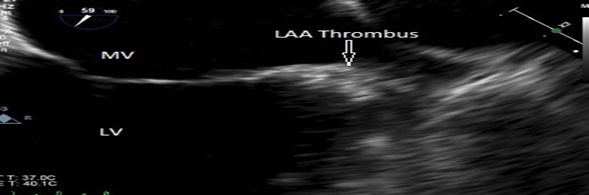 Figure 2: Transesophageal echocardiogram in the mid esophageal [60-degree] view showing left atrial appendage thrombus [arrow].
Figure 2: Transesophageal echocardiogram in the mid esophageal [60-degree] view showing left atrial appendage thrombus [arrow].
(LV= left ventricle, MV=mitral valve).
The cardioversion was cancelled due the left atrial appendage clot. His HR was eventually controlled with metoprolol and digoxin on the third day of hospitalization. He underwent a cardiac catheterization which revealed a focal myocardial bridge in the mid-left anterior descending artery and other arteries were shown to be patent. Patient was discharged on an outpatient regimen of metoprolol succinate 25 mg daily, lisinopril 5 mg daily and apixaban 5 mg bid. He was discharged on lifevest [wearable defibrillator] for prevention of sudden cardiac death for severe systolic dysfunction.
Transthoracic echocardiogram performed 3 months after initial presentation demonstrated mild left ventricular global hypokinesis with an ejection fraction of 45-50%, mild tricuspid and mitral regurgitation. Cardiac MRI performed 5 months after initial presentation demonstrated delayed subepicardial enhancement involving the basal, mid-anterolateral, and inferolateral walls with associated mild myocardial thinning and fibrosis with a left ventricular ejection fraction of 50%. Muscular dystrophy related subepicardial fibrosis was considered the primary diagnosis. The prior left atrial appendage clot was resolved (Figure 3).
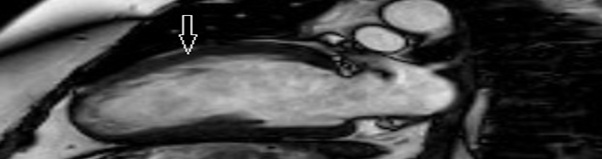 Figure 3: Cardiac MRI with delayed gadolinium enhancement showed subepicardial enhancement involving the basal, mid-anterolateral, and inferolateral walls with associated mild myocardial thinning and fibrosis [arrow].
Figure 3: Cardiac MRI with delayed gadolinium enhancement showed subepicardial enhancement involving the basal, mid-anterolateral, and inferolateral walls with associated mild myocardial thinning and fibrosis [arrow].
DIFFERENTIAL DIAGNOSIS
Cardiomyopathy from viral myocarditis, coronary artery disease, temporal arteritis and BMD associated myocardial dysfunction.
TREATMENT
He stopped wearing the Lifevest in three months after the repeat echocardiogram showed improvement in left ventricular function. He is currently managed on beta blockers, entresto [sacubitril/valsartan], spironolactone and on oral anticoagulation with apixaban.
OUTCOME AND FOLLOW-UP
The cause of his monocular blindness was determined to be due to cardiac emboli from the atrial flutter. He had developed severe systolic dysfunction from tachycardia induced cardiomyopathy which improved with rate control strategies. One year after his initial presentation, the patient complains of New York Heart Association Class II exertional shortness of breath without any associated chest pain, orthopnea, paroxysmal nocturnal dyspnea or pedal edema. The patient can walk a mile a day without any difficulty. He will be receiving annual vaccination with an inactivated influenza vaccine. He will be followed by his cardiologist with annual electrocardiograms, echocardiograms and 24 Hr Holter monitor.
DISCUSSION
Becker Muscular Dystrophy (BMD) is an X-linked recessive disorder resulting from mutations in the dystrophin gene located on chromosome Xp21.1. BMD affects one in every 18,450 male births [1,2]. It is characterized by progressive skeletal muscle failure with eventual cardiac involvement. In Duchenne Muscular Dystrophy (DMD), dystrophin is nearly absent whereas in Becker Muscular Dystrophy (BMD), dystrophin is present but reduced in size or amount. This leads to the characteristic rapidly progressive skeletal muscle disease in DMD and the more benign course in BMD [3]. The diagnosis of BMD is commonly established in early-late adolescence, with adult-onset diagnosis being rare.
Cardiomyopathy is the number one cause of death in patients with BMD. Dystrophin deficiency in the heart manifests as a cardiomyopathy. As the disease progresses, the myocardium fails to meet physiological demands and clinical heart failure develops. The failing myocardium is also at risk of life-threatening rhythm abnormalities. The pathoanatomical background of cardiomyopathy in BMD includes the replacement of dysfunctional cardiomyocytes and the intracardiac impulse generation and conduction system by fibrous tissue or fat. Extensive myocardial fibrosis in an early stage of the disease may be a surrogate marker for poor clinical outcomes [4]. The frequency of cardiac involvement in BMD is 60% to 75% with the average age of onset of cardiac involvement 28.7±7.1 years [5]. Different cardiac manifestations occur in BMD, ranging from very subtle signs to severe cardiomyopathy requiring cardiac transplant [6]. Most patients with cardiac involvement are asymptomatic and only up to one-third of patients with BMD develop dilated cardiomyopathy with concomitant heart failure.
While cardiac involvement is consistently present in BMD, its severity cannot be predicted by age, degree, duration of skeletal muscle weakness, type of mutation, or presence of cardiac symptoms, which are usually absent [7]. Although CK and CK-MB may be elevated in some cases, determination of CK or CK-MB is of little help in diagnosing cardiac involvement in BMD [8]. Cardiac involvement manifests as electrocardiographic abnormalities, hypertrophic cardiomyopathy, and dilation of the cardiac cavities with preserved systolic function, dilated cardiomyopathy or cardiac arrest. ECG abnormalities frequently found include sinus tachycardia at rest, atrial fibrillation, PQ shortening without delta wave, suggesting atriofascicular bands or accelerated conduction of the Atrioventricular (AV) node, intraventricular conduction delay with QRS broadening and a hypertrophy pattern [9,10].
There is no correlation between cardiac involvement and the severity of myopathy and is more prominent in patients than carriers [11]. Cardiac involvement of BMD was reported to be uncommon in patients under 16 years of age, whereas more than 70% of patients demonstrated pathologic cardiac findings by the age of 40 years [12,13]. Consensus guidelines recommend universal immunization of infants and children with pneumococcal vaccine, integrating 13-valent Pneumococcal Conjugate Vaccine (PCV13) with Pneumococcal Polysaccharide Vaccine (PPSV23) and annual vaccination with an inactivated influenza vaccine for all individuals with DMD and BMD six months of age and older, and all close contacts [14].
Historically, individuals with BMD have not been referred to a cardiac specialist until late in the disease, contributing to poor clinical outcomes. Furthermore, cardiac management has been challenging because the New York Heart Association classification of heart failure [15] relies on reduced exercise tolerance, a feature that in BMD arises from skeletal muscle and cardiac disease combined. The signs and symptoms of heart failure in the non-ambulatory individual are frequently subtle and overlooked. A proactive strategy of early diagnosis and treatment is essential to maximize duration and quality of life. Involvement of a cardiologist who is integrated into a multidisciplinary care team is recommended, given the complex decision making involved in managing BMD cardiomyopathy.
Irrespective of age, pharmacological therapy should be initiated with the onset of heart failure symptoms or when abnormalities such as depressed left ventricular ejection fraction, abnormal chamber dimensions, or the presence of myocardial fibrosis are noted on imaging studies (CMR or echocardiogram). Given the absence of dystrophin-specific targeted cardiac therapies, traditional treatment strategies for heart failure should be used including beta receptor antagonist, spironolactone and ACE inhibitors or ARBS [16]. Extraocular Muscles (EOM) are a few skeletal muscle groups that retain normal structure and function in both DMD and BMD. EOM’s ability to retain a normal structure and function in patients diagnosed with BMD depends on the indistinct features which protect it from the toxic effects of intracellular calcium, which is related to myofibril necrosis in absence of dystrophin [17]. No significant eye manifestations have been mentioned in patients with dystrophinopathies.
Once a diagnosis of BMD has been established, a comprehensive cardiac assessment and work-up should be performed. Cardiac involvement in BMD is progressive, thus regular surveillance and follow-up with transthoracic echocardiography is recommended for all patients with BMD, regardless of whether they are symptomatic or not. When cardiac involvement in BMD is recognized early, appropriate therapy may be applied early, resulting in a more favorable outcome. Involvement of a cardiologist who is integrated into a multidisciplinary care team is recommended, given the complex decision making involved in managing BMD cardiomyopathy. The case herein demonstrates a patient with late-onset BMD who subsequently developed rapidly progressive acute congestive heart failure with evidence of cardiomyopathy. His presentation was atypical as he developed transient monocular blindness due to cardioembolic etiology from atrial fibrillation. This is the first reported case of a patient with BMD presenting with ocular symptoms. He developed tachymyopathy due to atrial flutter with rapid ventricular rate. His cardiomyopathy improved with rate control strategy for the atrial flutter and goal directed medication therapy for congestive heart failure including beta receptor antagonist, entresto and spironolactone. This patient had previously been asymptomatic and displayed no indication of cardiac involvement. This case demonstrates the unpredictable and insidious course of cardiomyopathic dysfunction in muscular dystrophies. Diligence in initial cardiac assessment and early preemptive therapeutic measures can help reduce the progression of cardiac involvement seen in muscular dystrophies.
LEARNING POINTS/TAKE HOME MESSAGES
• The frequency of cardiac involvement in Becker Muscular Dystrophy (BMD) is 60% to 75%
• Comprehensive cardiac assessment and work-up should be performed as soon as a diagnosis of BMD has been established
• When cardiac involvement in BMD is recognized early, appropriate interventional therapy may be applied early, resulting in a more favorable outcome
• It is imperative to remember that patients can have an atypical presentation with transient vision loss as the presenting symptom
REFERENCES
- Yilmaz A, Sechtem U (2012) Cardiac involvement in muscular dystrophy: Advances in diagnosis and therapy. Heart 98: 420-429.
- Ho R, Nguyen ML, Mather P (2016) Cardiomyopathy in becker muscular dystrophy: Overview. World J Cardiol 8: 356-361.
- Nigro G, Comi LI, Politano L, Limongelli FM, Nigro V, et al. (1995) Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle Nerve 18: 283-291.
- Nigro G, Politano L, Nigro V, Petretta VR, Comi LI (1994) Mutation of dystrophin gene and cardiomyopathy. Neuromuscul Disord 4: 371-379.
- Rajdev A, Groh WJ (2015) Arrhythmias in the Muscular Dystrophies. Card Electrophysiol Clin 7: 303-308.
- Srinivasan R, Hornyak JE, Badenhop DT, Koch LG (2005) Cardiac rehabilitation after heart transplantation in a patient with Becker's muscular dystrophy: A case report. Arch Phys Med Rehabil 86: 2059-2061.
- Saito M, Kawai H, Akaike M, Adachi K, Nishida Y, et al. (1996) Cardiac dysfunction with Becker muscular dystrophy. Am Heart J 132: 642-647.
- Finsterer J, Stöllberger C (2008) Cardiac involvement in Becker muscular dystrophy. Can J Cardiol 24: 786-792.
- Finsterer J, Stöllberger C (2003) The heart in human dystrophinopathies. Cardiology 99: 1-19.
- Miyashita H, Ikeda U, Shimada K, Natsume T, Arahata K (1993) Becker muscular dystrophy with early manifestation of left heart failure. Intern Med 32: 408-411.
- Melacini P, Fanin M, Danieli GA, Fasoli G, Villanova C, et al. (1993) Cardiac involvement in Becker muscular dystrophy. J Am Coll Cardiol 22: 1927-1934.
- Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, et al. (1996) Myocardial involvement is very frequent among patients affected with subclinical Becker's muscular dystrophy. Circulation 94: 3168-3175.
- Kaspar RW, Allen HD, Ray WC, Alvarez CE, Kissel JT, et al. (2009) Analysis of dystrophin deletion mutations predicts age of cardiomyopathy onset in becker muscular dystrophy. Circ Cardiovasc Genet 2: 544-551.
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, et al. (2018) Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol 17: 445-455.
- American Heart Association (2017) Classes of heart failure. American Heart Association, Dallas, Texas, USA.
- Raman SV, Hor KN, Mazur W, He X, Kissel JT, et al. (2017) Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: Results of a two-year open-label extension trial. Orphanet J Rare Dis 12: 39.
- Evliyaoglu F, Burakgazi AZ (2015) Ocular findings in muscular dystrophies. Journal of Medicine and Medical Sciences 6: 234-242.
Citation: Finer C, Gammon T, Bhandare D (2020) Transient Monocular Vision Loss in a Patient with Becker Muscular Dystrophy. J Non Invasive Vasc Invest 5: 022.
Copyright: © 2020 Christa Finer, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.