
Journal of Otolaryngology Head & Neck Surgery Category: Clinical
Type: Research Article
Transoral Robotic Surgery for Squamous Cell Carcinoma of the Oropharynx. Outcomes of the First Multicentric Series in Spain
*Corresponding Author(s):
David Virós PorcunaOtolaryngology Section Head And Neck Surgery, Hospital Germans TriasiPujol, Badalona, Spain
Tel:+34 651167837,
Email:davidviros@gmail.com
Received Date: May 03, 2019
Accepted Date: May 22, 2019
Published Date: May 29, 2019
Abstract
Background
Transoral Robotic Surgery (TORS) has become one of the mainstays of oropharyngeal cancer treatment in the last decade. However its introduction in Spain has been very limited. We present the first clinical case series study in our country.
The objective is to analyse the outcomes of this approach in our country in order to contribute to the definition of the role of this technique.
Methods
Retrospective analysis of prospectively collected data from patients receiving TORS for oropharyngeal cancer in three centers. Management protocols, including the reconstructive scheme after TORS, are reviewed.
Results
Eighty-three patients receiving TORS, 87.8% as a primary treatment and 12.2% as a salvage procedure, were included; 55.4% were advanced stages. Global complication rate was 13.4%, being bleeding the most frequent (9.8%). Reconstructive procedures were associated in 14.4% of cases. Normal swallowing was achieved in 82.4% of patients at three months after surgery. Patients treated with TORS as a primary treatment did not need complementary treatment in 56.6% of cases.
Conclusion
We found TORS to be a safe and effective approach in the treatment of oropharyngeal cancer. In our experience the practical contribution would be the reduction of the need for chemoradiation which could explain thefunctional outcomes.
Transoral Robotic Surgery (TORS) has become one of the mainstays of oropharyngeal cancer treatment in the last decade. However its introduction in Spain has been very limited. We present the first clinical case series study in our country.
The objective is to analyse the outcomes of this approach in our country in order to contribute to the definition of the role of this technique.
Methods
Retrospective analysis of prospectively collected data from patients receiving TORS for oropharyngeal cancer in three centers. Management protocols, including the reconstructive scheme after TORS, are reviewed.
Results
Eighty-three patients receiving TORS, 87.8% as a primary treatment and 12.2% as a salvage procedure, were included; 55.4% were advanced stages. Global complication rate was 13.4%, being bleeding the most frequent (9.8%). Reconstructive procedures were associated in 14.4% of cases. Normal swallowing was achieved in 82.4% of patients at three months after surgery. Patients treated with TORS as a primary treatment did not need complementary treatment in 56.6% of cases.
Conclusion
We found TORS to be a safe and effective approach in the treatment of oropharyngeal cancer. In our experience the practical contribution would be the reduction of the need for chemoradiation which could explain thefunctional outcomes.
Keywords
Head and Neck Cancer; Oropharyngeal Neoplasms; Radiotherapy Dosage; Reconstructive Surgical Procedures; Robotic Surgical Procedures
ABBREVIATIONS
RT: Radiation therapy;
CRT: Chemoradiotherapy;
HPV: Human Papillomavirus;
TORS: Trans Oral Robotic Surgery;
RT 70Gy: Radiotherapy 66-70 Gy dose;
RT 60Gy: Radiotherapy 54-63 Gy dose;
FEES: Fiberoptic Endoscopic Evaluation of Swallowing;
PCR: Polymerase chain reaction;
IMRT: Intensity-Modulated Radiation Therapy.
CRT: Chemoradiotherapy;
HPV: Human Papillomavirus;
TORS: Trans Oral Robotic Surgery;
RT 70Gy: Radiotherapy 66-70 Gy dose;
RT 60Gy: Radiotherapy 54-63 Gy dose;
FEES: Fiberoptic Endoscopic Evaluation of Swallowing;
PCR: Polymerase chain reaction;
IMRT: Intensity-Modulated Radiation Therapy.
BACKGROUND
Due to the morbidity caused by surgical procedures in this area, non-surgical treatments have been widely used for head and neck cancer over the past 30 years. Although there are no randomised prospective comparative studies that show the superiority of Radiation Therapy (RT) over open surgery, the former was adopted as the most common standard treatment of these tumours [1]. During the early 2000s, after studies showing improved survival with the addition of chemotherapy, Chemoradiation (CRT) became the standard [2,3]. Nevertheless the morbidity profile related to these treatments resulted in an increase in acute and late toxicities. The meta-analysis of Machtay, et al., [4] showed that CRT as a primary treatment left severe toxicity in more than 43% of cases.
The incidence of squamous cell carcinoma of the larynx, oral cavity, and hypopharynx has decreased in recent years, whereas that of squamous cell carcinoma of the oropharynx has been steadily increasing [5]. This seems related to epidemiological changes due to Human Papillomavirus (HPV) infection [6]. The HPV has been confirmed as an etiologic agent for oropharyngeal carcinomas. Its incidence is very high in northern European countries, as well as in the USA [7,8,9,10,11]. In Spain, as in other Mediterranean countries, the incidence is possibly lower, although there is currently very little evidence in this regard [12,13].
The response to treatments of these HPV-related tumours has been found to be very different from squamous cell carcinomas mediated by classical etiological factors (related to toxic habits, particularly tobacco and alcohol) [14,15,16]. Clearly, HPV mediated carcinoma shows a better prognosis, even in those patients who also have toxic habits. This improvement in survival has been reported both in those patients undergoing surgical treatment [17,18,19] and primary treatment based on RT [20,21,22].
Since the development of laser microsurgery [23], transoral surgery has evolved with technological progress. Possibly the most important single factor in recent years has been the introduction of endoscopes that have supported new surgical techniques either with the use of laparoscopic tools [24] or with robotic platforms [25]. Oropharyngeal surgery has moved towards transoral approaches to reduce the morbidity of open approaches, which have been associated with a complication rate of 10 to 60%, including dysphagia, temporomandibular malocclusion, aesthetic deformity, and fistula [26,27]. The transoral choicecarries a benefit in efficiency and functionality compared to alternative surgical approaches and other non-surgical treatment options [28,29]. In addition, these approaches reduce the need for reconstructive procedures [30], while maintaining the oncological results [31,32].
Currently there is a need to define the exact position of transoral approaches, and particularly of Transoral Robotic Surgery (TORS) in the treatment of head and neck cancer, especially in squamous carcinoma of the oropharynx. Unlike other countries, TORS has had a limited expansion in Spain. The objective of this paper is to present the first outcomes in our country and analyze its role through a multi centric series.
The incidence of squamous cell carcinoma of the larynx, oral cavity, and hypopharynx has decreased in recent years, whereas that of squamous cell carcinoma of the oropharynx has been steadily increasing [5]. This seems related to epidemiological changes due to Human Papillomavirus (HPV) infection [6]. The HPV has been confirmed as an etiologic agent for oropharyngeal carcinomas. Its incidence is very high in northern European countries, as well as in the USA [7,8,9,10,11]. In Spain, as in other Mediterranean countries, the incidence is possibly lower, although there is currently very little evidence in this regard [12,13].
The response to treatments of these HPV-related tumours has been found to be very different from squamous cell carcinomas mediated by classical etiological factors (related to toxic habits, particularly tobacco and alcohol) [14,15,16]. Clearly, HPV mediated carcinoma shows a better prognosis, even in those patients who also have toxic habits. This improvement in survival has been reported both in those patients undergoing surgical treatment [17,18,19] and primary treatment based on RT [20,21,22].
Since the development of laser microsurgery [23], transoral surgery has evolved with technological progress. Possibly the most important single factor in recent years has been the introduction of endoscopes that have supported new surgical techniques either with the use of laparoscopic tools [24] or with robotic platforms [25]. Oropharyngeal surgery has moved towards transoral approaches to reduce the morbidity of open approaches, which have been associated with a complication rate of 10 to 60%, including dysphagia, temporomandibular malocclusion, aesthetic deformity, and fistula [26,27]. The transoral choicecarries a benefit in efficiency and functionality compared to alternative surgical approaches and other non-surgical treatment options [28,29]. In addition, these approaches reduce the need for reconstructive procedures [30], while maintaining the oncological results [31,32].
Currently there is a need to define the exact position of transoral approaches, and particularly of Transoral Robotic Surgery (TORS) in the treatment of head and neck cancer, especially in squamous carcinoma of the oropharynx. Unlike other countries, TORS has had a limited expansion in Spain. The objective of this paper is to present the first outcomes in our country and analyze its role through a multi centric series.
METHODS
This study is based on a retrospective analysis of oncological data collected prospectively from patients undergoing TORS with the da Vinci robotic system (Intuitive Surgical Inc) for squamous cell carcinoma of the oropharynx, in three different centers that currently gather the majority of TORS cases in the country. Participating institutions include Hospital Universitario Rey Juan Carlos (Madrid), Hospital Universitari Son Espases (Mallorca), Hospital Universitari Germans TriasiPujol (Badalona/Barcelona). The earliest TORS programme in the 3 centres was started in July 2013, and in year 2015 and 2017 respectively in the other two.
All the patients received clear and accurate information about the procedure they would be undergoing. Informed consent was obtained from all individual participants included in the study.
Inclusion criteria were patients undergoing TORS for oropharyngeal cancer in those centres until January 2018. The treatment scheme for every patient was decided after consultation with a multidisciplinary tumour board on each institution. The treatment protocol for these patients is based on anatomoclinical features of the tumour and on patient’s clinical conditions. Thus, patients with tumours classified as T1-T2-T3 [33] with a reasonable prospect to achieve free margins, and N0 or N + without suspected extracapsular spread [34], are considered for TORS.
Adjuvant treatment is evaluated according to pathology. Adverse features like positive margin or extracapsular spread are treated with 66 to 70Gy Radiotherapy (RT 70Gy) associated or not with systemic therapy (CRT). Locally advanced tumours (T3-T4) with negative margins usually were treated with 54 to 63 Gy RT (RT 60Gy).
The alternative standard treatment for these patients would be radical RT (70Gy) or CRT. Surgical treatment was also proposed in those patients who have received prior RT in the head and neck area and cannot be reirradiated with radical doses, or in patients not suitable for CRT due to medical conditions. Some of them might not be candidates for a fully transoral approach due to tumour extension, but a combined TORS and transcervical approach according to oncological extension was used to reduce surgical morbidity. Our reconstructive algorithm (Figure 1) is based on the local extension of the tumour, the depth of the surgical defect and whether prior therapy was received. We performed reconstruction on those patients with carotid exposure and oro cervical communication, and in some instances on pretreated patients in order to avoid bleeding complications.
All the patients received clear and accurate information about the procedure they would be undergoing. Informed consent was obtained from all individual participants included in the study.
Inclusion criteria were patients undergoing TORS for oropharyngeal cancer in those centres until January 2018. The treatment scheme for every patient was decided after consultation with a multidisciplinary tumour board on each institution. The treatment protocol for these patients is based on anatomoclinical features of the tumour and on patient’s clinical conditions. Thus, patients with tumours classified as T1-T2-T3 [33] with a reasonable prospect to achieve free margins, and N0 or N + without suspected extracapsular spread [34], are considered for TORS.
Adjuvant treatment is evaluated according to pathology. Adverse features like positive margin or extracapsular spread are treated with 66 to 70Gy Radiotherapy (RT 70Gy) associated or not with systemic therapy (CRT). Locally advanced tumours (T3-T4) with negative margins usually were treated with 54 to 63 Gy RT (RT 60Gy).
The alternative standard treatment for these patients would be radical RT (70Gy) or CRT. Surgical treatment was also proposed in those patients who have received prior RT in the head and neck area and cannot be reirradiated with radical doses, or in patients not suitable for CRT due to medical conditions. Some of them might not be candidates for a fully transoral approach due to tumour extension, but a combined TORS and transcervical approach according to oncological extension was used to reduce surgical morbidity. Our reconstructive algorithm (Figure 1) is based on the local extension of the tumour, the depth of the surgical defect and whether prior therapy was received. We performed reconstruction on those patients with carotid exposure and oro cervical communication, and in some instances on pretreated patients in order to avoid bleeding complications.
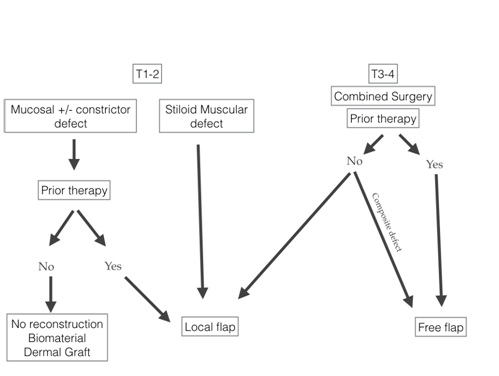
Figure 1: Reconstructive algorithm after transoral approach.
Perioperative management was fairly uniform between the centres. In general, prophylactic tracheostomy was not prescribed. Those patients who showed edema at the end of the surgery or doubts about their airway safety remained intubated (orotraqueal or nasotraqueal) for 12h-24h; if the situation did not improve, a temporary tracheostomy was considered.
Prior to hospital discharge, a functional assessment of swallowing was performed by Fiberoptic Endoscopic Evaluation of Swallowing (FEES) and / or videofluoroscopy, and patients were assessed in the dysphagia unit of each centre.
The method to identify HPV varied with time. Polymerase Chain Reaction (PCR) analysis was preferably used, taking advantage of the previous lab circuit for the uterine cervix until 2016, and later and following current recommendations it is based on p16 imunohistochemistry and PCR combination detection [35].
We analysed tumour extension according to TNM staging, surgical margins, associated procedures, nasogastric and/or gastrostomy tube, hospital stay, complications, need for complementary treatment and swallowing function. The Chi-square test and the T test were used to compare qualitative and quantitative variables, respectively. Survival curves were estimated using the Kaplan-Meier actuarial method.
RESULTS
A total of 86 patients receiving TORS for oropharyngeal carcinoma were included. The conversion rate was 3.5% due to 3 cases who had to be converted to open surgery due to anatomical conditions which made tumour exposure impossible with FK-WO (Olympus Corp) or Crowe-Davis mouth gag.
Those 3 patients were excluded of the subsequent TORS outcomes analysis.
Patients consisted of 67 men and 16 women, with a mean age of 62 years (SD: 8.95). HPV was detected in 29% of the cases (n=25), in 60% by PCR and p16 and in 40% by p16. Clinical staging is shown in table 1. The correlation between the primary site and local stage is shown in figure 2.
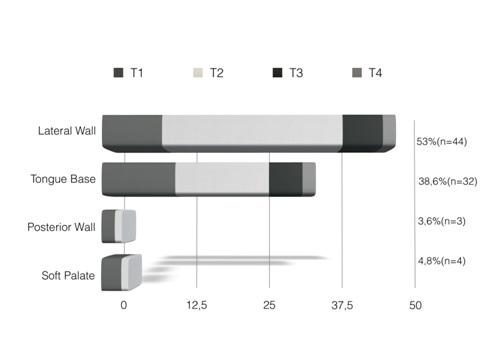
Figure 2: T stage according to Oropharyngeal location.
Those 3 patients were excluded of the subsequent TORS outcomes analysis.
Patients consisted of 67 men and 16 women, with a mean age of 62 years (SD: 8.95). HPV was detected in 29% of the cases (n=25), in 60% by PCR and p16 and in 40% by p16. Clinical staging is shown in table 1. The correlation between the primary site and local stage is shown in figure 2.

Figure 2: T stage according to Oropharyngeal location.
|
|
|
|
N2 |
|
|
||
|
|
N0 |
N1 |
N2a |
N2b |
N2c |
N3 |
Total |
|
T1 |
13 |
3 |
7 |
0 |
0 |
2 |
25 |
|
T2 |
24 |
5 |
4 |
7 |
1 |
2 |
43 |
|
T3 |
6 |
1 |
0 |
2 |
1 |
1 |
11 |
|
T4 |
2 |
2 |
0 |
0 |
0 |
0 |
4 |
|
Total |
45 |
11 |
11 |
9 |
2 |
5 |
83 |
Seventy two (87.8%) patients were treated with TORS as a primary surgery. Eleven (12.2%) previously received RT or CRT and underwent TORS as a salvage procedure. Unilateral neck dissection was performed in 19.3% (n = 16) and bilateral neck dissection in 25.3% (n = 21) in the same surgical procedure. Reconstructive procedures were associated in 14.4% of the cases (n=12). When primary or salvage surgery was considered, reconstruction was performed in 9.7% of first line surgery cases (6 regional flaps and 1 free flap), and in 45% of salvage cases (3 free flaps and 2 regional flaps). Temporary tracheostomy was required in 7 cases (8.4%). Free resection margins were achieved on 85% of the cases (n=71), close margins appeared in 10% (n=8) and affected margins in 5% (n=4).
The complication rate was 13.4%. Bleeding was the most frequent complication (9.8%) and required revision hemostatic surgery in 91% of cases. 1.2% of the patients suffered postoperative dyspnea, 2.4% pharyngocutaneous fistula (managed conservatively, without surgery in all of the cases), and 1.2% death due to bleeding aspiration. Complications appeared to be associated with pT staging, although this did not reach statistical significance (p=0.084.)
Mean hospital stay was 8.3 days (DE: 8.0) with a median of 7 days. The duration of the hospital stay was not associated either with salvage surgery, staging or T category, but a statistically significant association was found with the presence of postoperative complications (p=0.001).
At the time of hospital discharge, functional assessment of swallowing by FEES was normal in 48.8% of the patients. Up to 28.8% received diet adaptation, 13.3% swallowing rehabilitation and 8.8% a nasogastric feeding tube. At 3 months, swallowing evaluation was normal in 82.4% of the patients, while 6.8% kept diet adaptation, 4% swallowing rehabilitation and 6.8% a feeding tube (gastrostomy). The mean duration of feeding tube postoperatively was 7,1 days.
Specific swallowing care was not associated with T stage, with primary or salvage surgery.
Among patients who received primary surgery, 56.6% did not need complementary treatment (“TORS alone” cases). RT 60Gy was given to 13.2%, CRT to 28.9% and RT 70Gy to 1.2%. We analysed the need for adjuvant treatment according to the initial staging (Figure 3).
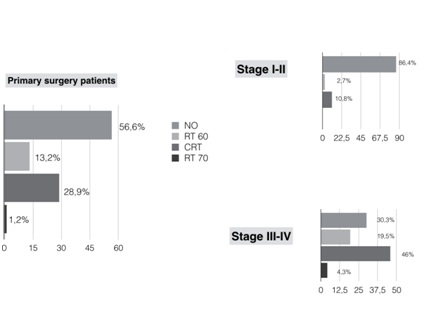
Figure 3: Complementary treatment on primary surgery patients.
For early stages (I,II, n=37) TORS alone cases were 86.4%, while for advanced stages (III,IV) were 30.3% of the cases. The need for complementary treatment was associated with the initial staging (p=0.001).
Mean follow-up was 17.7 months (DE: 14.4m). Overall2 years survival was 88.8% and specific 2 years survival was 91,4%. Two years Survival for patients undergoing salvage surgery was 66,7 % while for primary surgery patients this was 91,5% (p=0.002) (Figure 4).
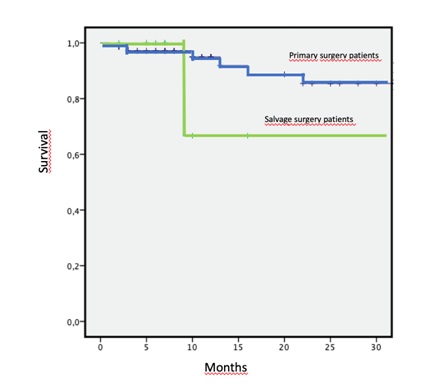
Figure 4: Overall 2 years survival for primary surgery and salvage surgery patients.
DISCUSSION
Regardless of the surgical approach that is chosen for the treatment of squamous cell carcinoma of the oropharynx, the principles of complete resection with minimum free oncological safe margins should be respected; usually, when a minimally invasive approach is used, a satisfactory functional outcome is also expected. Complementary CRT should not be considered as a solution to insufficient surgery. Also, a significant reduction in the dose of adjuvant treatment should be obtained compared to primary treatment with CRT [36]. When TORS is considered, a careful assessment of the patient should be made, focusing on the examination of the mouth opening and dentition, the size of the base of the tongue and jaw, the cervical length and the atlanto-occipital extensión [37]. Contraindications for TORS should be ruled out: Involvement of the base of the skull, the jaw or the carotid artery, or unresectable lymphadenopathies [38]. An appropriate access to these tumours requires that the tumourcan be completely visualised with the endoscope so that a complete circumferential resection can be done, while controlling the cervical neurovascular structures in the deep limit of the resection.
When assessing the need for complementary treatment, neck status should be evaluated. Cases with pN0 staging would not need adjuvant treatment after neck dissection, while those N + with capsular rupture, size greater than 6 cm or more than 4 positive nodes would be candidates for adjuvant CRT, decreasing the potential benefit of TORS as primary treatment.
Stages I-II should be offered a unimodal treatment. In this group of patients, the treatment options would be TORS versus radical RT (which should be Intensity-Modulated Radiation Therapy, IMRT as a standard). For patients in stages III and IV, treatment options would be TORS versus CRT. In general, multimodal treatment is considered for advanced stages. In this situation, the reason for starting with TORS would be to decrease the need for adjuvant treatment, which means reducing the dose of RT or avoiding the need for concurrent systemic therapy.
Stages I-II patients received TORS alone in 86.5% of cases. For advanced stages we could reduce or spare adjuvant treatment in 53.8% of patients.
Some studies analyse the need for adjuvant treatment after TORS. Lorincz, et al., [39] present 50 patients T1-2 N0 and N+ without suspected extra capsular spread 40% of the patients received “TORS alone” without adjuvant treatment and in 34% either the dose of RT was reduced or chemotherapy avoided. That means that in 74% of the patients the need of adjuvant treatment was decreased or avoided. Smith, et al.,[36] prospectively analyse 44 patients stages I to IV. 52% required adjuvant treatment (21% RT and 31% CRT). This need for treatment was associated with T and N categories. Nevertheless, 18% of the patients were re-staged after surgery. Local control and survival in the surgery group were better, although without statistical significance. In our series T was changed in 9.6% and global stage in 14.5% (mostly because of discrepancies between radiologic and pathologic examination of the neck).
Our study cohort showed a 91.4% specific disease survival. One of the limitations of the study was the limited follow-up time (mean 17.7 months). Expected survival, according to published data is between 83% and 96% for T1 and between 54% and 92% for T2. TORS studies show better survival results, although follow-up is still shorter (with an average of 2 years) [40].
Although TORS is a minimally invasive surgical approach which functional outcomes overcome those of open surgery, it is also associated with potentially severe complications. Bleeding is a particular concern due to the site because it can be aspirated and end in death by asphyxia. The incidence of bleeding complications described in the literature ranges from 1.5% to 11% [41]. In patients with salvage surgery this can be as high as 19%. Another complication is the pharyngo-cutaneous fistula, particularly when neck dissection is done simultaneously.
Our complication rate was 13.4% and hemorrhagic complications were 9.8%. These did not present a statistical relationship with salvage surgery. In those patients undergoing salvage surgery, the decision threshold to go for a reconstructive procedure with the aim of reducing possible complications was reduced compared to those treated with TORS in the first line of treatment. While on first line surgery we performed reconstructive procedures in 9.7% of the cases, in salvage surgery it was 45%. We found a statistical trend in the relationship between complications and category T that denotes greater resection.
Favourable functional outcomes with normal swallowing in the long term vary between 70% and 100% [42]. Regarding feeding tube dependence Setton, et al.,[43] presented a multicentric study with 2,300 patients treated with IMRT with an incidence between 3.7% and 4.4% depending on the stage, although numbers vary in a wide range from 2% to 20% one year after treatment. In TORS series the percentage is consistently between 0% and 9% at one year [44]. Furthermore there are several studies comparing quality of life for TORS patients (with or without RT) versus primary RT or CRT, and no differences were found except for dysphagia where there is a tendency to be subjectively better in patients treated with surgery [45,46,47].
This study is the largest reported oropharyngeal cancer TORS cohort in our country, and joins the learning curve and later experience of three centres. Probably it could be a weakness of our study because of the heterogeneity between centres. On the other hand, It could prove that TORS is a reproducible technique and with good oncological and functional results in selected cases.
We think this can support the shifting paradigm for oropharyngeal cancer from chemoradiotherapy to surgery. This concept is not broadly accepted yet in our nation.
When assessing the need for complementary treatment, neck status should be evaluated. Cases with pN0 staging would not need adjuvant treatment after neck dissection, while those N + with capsular rupture, size greater than 6 cm or more than 4 positive nodes would be candidates for adjuvant CRT, decreasing the potential benefit of TORS as primary treatment.
Stages I-II should be offered a unimodal treatment. In this group of patients, the treatment options would be TORS versus radical RT (which should be Intensity-Modulated Radiation Therapy, IMRT as a standard). For patients in stages III and IV, treatment options would be TORS versus CRT. In general, multimodal treatment is considered for advanced stages. In this situation, the reason for starting with TORS would be to decrease the need for adjuvant treatment, which means reducing the dose of RT or avoiding the need for concurrent systemic therapy.
Stages I-II patients received TORS alone in 86.5% of cases. For advanced stages we could reduce or spare adjuvant treatment in 53.8% of patients.
Some studies analyse the need for adjuvant treatment after TORS. Lorincz, et al., [39] present 50 patients T1-2 N0 and N+ without suspected extra capsular spread 40% of the patients received “TORS alone” without adjuvant treatment and in 34% either the dose of RT was reduced or chemotherapy avoided. That means that in 74% of the patients the need of adjuvant treatment was decreased or avoided. Smith, et al.,[36] prospectively analyse 44 patients stages I to IV. 52% required adjuvant treatment (21% RT and 31% CRT). This need for treatment was associated with T and N categories. Nevertheless, 18% of the patients were re-staged after surgery. Local control and survival in the surgery group were better, although without statistical significance. In our series T was changed in 9.6% and global stage in 14.5% (mostly because of discrepancies between radiologic and pathologic examination of the neck).
Our study cohort showed a 91.4% specific disease survival. One of the limitations of the study was the limited follow-up time (mean 17.7 months). Expected survival, according to published data is between 83% and 96% for T1 and between 54% and 92% for T2. TORS studies show better survival results, although follow-up is still shorter (with an average of 2 years) [40].
Although TORS is a minimally invasive surgical approach which functional outcomes overcome those of open surgery, it is also associated with potentially severe complications. Bleeding is a particular concern due to the site because it can be aspirated and end in death by asphyxia. The incidence of bleeding complications described in the literature ranges from 1.5% to 11% [41]. In patients with salvage surgery this can be as high as 19%. Another complication is the pharyngo-cutaneous fistula, particularly when neck dissection is done simultaneously.
Our complication rate was 13.4% and hemorrhagic complications were 9.8%. These did not present a statistical relationship with salvage surgery. In those patients undergoing salvage surgery, the decision threshold to go for a reconstructive procedure with the aim of reducing possible complications was reduced compared to those treated with TORS in the first line of treatment. While on first line surgery we performed reconstructive procedures in 9.7% of the cases, in salvage surgery it was 45%. We found a statistical trend in the relationship between complications and category T that denotes greater resection.
Favourable functional outcomes with normal swallowing in the long term vary between 70% and 100% [42]. Regarding feeding tube dependence Setton, et al.,[43] presented a multicentric study with 2,300 patients treated with IMRT with an incidence between 3.7% and 4.4% depending on the stage, although numbers vary in a wide range from 2% to 20% one year after treatment. In TORS series the percentage is consistently between 0% and 9% at one year [44]. Furthermore there are several studies comparing quality of life for TORS patients (with or without RT) versus primary RT or CRT, and no differences were found except for dysphagia where there is a tendency to be subjectively better in patients treated with surgery [45,46,47].
This study is the largest reported oropharyngeal cancer TORS cohort in our country, and joins the learning curve and later experience of three centres. Probably it could be a weakness of our study because of the heterogeneity between centres. On the other hand, It could prove that TORS is a reproducible technique and with good oncological and functional results in selected cases.
We think this can support the shifting paradigm for oropharyngeal cancer from chemoradiotherapy to surgery. This concept is not broadly accepted yet in our nation.
CONCLUSION
TORS has an important and expanding role in the treatment of oropharyngeal squamous cell carcinoma. Good oncologic and functional outcomes are found across three centres in the earliest series in our country, both as a first line treatment and as a salvage surgery.
REFERENCES
- Urban D, Corry J, Rischin D (2014) What is the Best Treatment for patients with human papillomavirus-positive and - negative oropharyngeal cancer? Cancer 120: 1462-1470.
- Pignon JP, le Maître A, Bourhis J (2007) Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): An update. Int J Radiat Oncol Biol Phys 69: 112-114.
- Pignon JP, le Maître A, Maillard E, Bourhis J (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 92: 4-14.
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, et al. (2008) Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 26: 3582-3589.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML (2008) Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26: 612-619.
- Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, et al. (2008) Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998-2003. Cancer 113: 2901-2909.
- Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, et al. (2009) Incidence of Human Papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer 125: 362-366.
- Lundberg M, Leivo I, Saarilahti K, Mäkitie AA, Mattila PS (2011) Increased incidence of oropharyngeal cancer and p16 expression. Acta Otolaryngol 131: 1008-1011.
- Blomberg M, Nielsen A, Munk C, Kjaer SK (2011) Trends in head and neck cancer incidence in Denmark, 1978-2007: Focus on human papilomavirus associated sites. Int J Cancer 129: 733-741.
- Garnaes E, Kiss K, Andersen L, Therkildsen MH, Franzmann MB, et al. (2014) A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000-2010: The largest registry-based study to date. Int J Cancer 136: 2196-2203.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- Rodrigo JP, Heideman DA, García-Pedrero JM, Fresno MF, Brakenhoff RH, et al. (2014) Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009). Int J Cancer 134: 487-92.
- Cerezo L, de la Torre A, Hervás A, Ruiz A, Liñán O, et al. (2014) Oropharyngeal cancer related to Human Papilloma Virus: Incidence and prognosis in Madrid, Spain. Clin Transl Oncol 16: 301-306.
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709-720.
- Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E (2000) Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int J Cancer. 89: 300-304.
- Semrau R, Duerbaum H, Temming S, Huebbers C, Stenner M, et al. (2013) Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head Neck 35: 1339-1344.
- Blanco RG, Fakhry C, Ha PK, Ryniak K, Messing B, et al. (2013) Transoral robotic surgery experience in 44 cases. J Laparoendosc Adv Surg Tech A 23: 900-907.
- Cohen MA, Weinstein GS, O'Malley BW Jr, Feldman M, Quon H (2011) Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck 33: 573-580.
- Olsen SM, Moore EJ, Laborde RR, Garcia JJ, Janus JR, et al. (2013) Transoral surgery alone for human-papillomavirus-associated oropharyngeal squamous cell carcinoma. Ear Nose Throat J 92: 76-83.
- Broglie MA, Soltermann A, Rohrbach D, Haile SR, Pawlita M, et al.(2013) Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity-modulated chemoradiation. Head Neck 35: 1698-1706.
- Nichols AC, Faquin WC, Westra WH, Mroz EA, Begum S, et al. (2009) HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol e Head Neck Surg 140: 228-234.
- Shoushtari A, Meeneghan M, Sheng K, Moskaluk CA, Thomas CY, et al. (2010) Intensity-modulated radiotherapy outcomes for oropharyngeal squamous cell carcinoma patients stratified by p16 status. Cancer 116: 2645-2754.
- Steiner W (1988) Experience in endoscopic laser surgery of malignant tumours of the upper aero-digestive tract. Adv Otorhinolaryngol 39: 135-144.
- Fernández-Fernández MM, Montes-Jovellar L, Parente Arias PL, Ortega Del Alamo P (2015) TransOral endoscopic UltraSonic Surgery (TOUSS): A preliminary report of a novel robotless alternative to TORS. Arch Otorhinolaryngol 272: 3785-3791.
- O’Malley BW Jr, Weinstein GS, Snyder W, Hockstein NG (2006) Transoral Robotic Surgery (TORS) for base of tongue neoplasms. Laryngoscope 116: 1465-1472.
- Tsue TT, Desyatnikova SS, Deleyiannis FW, Futran ND, Stack BC Jr, et al. (1997) Comparison of cost and function in reconstruction of the posterior oral cavity and oropharynx. Free vs pedicled soft tissue transfer. Arch Otolaryngol Head Neck Surg 123: 731-737.
- Moore EJ, Olsen KD, Kasperbauer JL (2009) Transoral robotic surgery for oropharyngeal squamous cell carcinoma: A prospective study of feasibility and functional outcomes. Laryngoscope 119: 2156-2164.
- Leonhardt FD, Quon H, Abrahão M, O'Malley BW Jr, Weinstein GS (2012) Transoralroboticsurgery for oropharyngeal carcinoma and its impact on patient-reportedquality of life and function. Head Neck 34: 146-154.
- Park YM, Kim WS, Byeon HK, Lee SY, Kim SH (2013) Oncological and functional outcomes of transoral robotic surgery for oropharyngeal cancer. Br J Oral Maxillofac Surg, 51: 408-412.
- Zafereo ME, Weber RS, Lewin JS, Roberts DB, Hanasono MM ( 2010 ) Complications and functional outcomes following complex oropharyngeal reconstruction. Head NeckAug 32: 1003-11.
- Williams CE, Kinshuck AJ, Derbyshire SG, Upile N, Tandon S, et al. (2014) Transoral laser resection versus lip-split mandibulotomy in the management of oropharyngeal squamous cell carcinoma (OPSCC): A case match study. Eur Arch Otorhinolaryngol 271: 367-372.
- White H, Ford S, Bush B, Holsinger FC, Moore E, et al. (2013) Salvage surgery for recurrent cancers of the oropharynx: Comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg 139: 773-778.
- Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, et al. (2017) Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 67: 122-137.
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, et al. (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 67: 93-99.
- Mena M, Taberna M, Tous S, Marquez S, Clavero O, et al. (2018) Double positivity for HPV-DNA/p16ink4a is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral Oncol 78: 137-144.
- Smith RV, Schiff BA, Garg M, Haigentz M (2015) The impact of transoral robotic surgery on the overall treatment of oropharyngeal cancer patients. Laryngoscope 10: 1-15.
- Moore EJ, Olsen SM, Laborde RR, García JJ, Walsh FJ, et al. (2012) Long-term functional and oncologic results of transoral roboticsurgery for oropharyngealsquamouscell carcinoma. Mayo ClinProc 87: 219-225.
- de Almeida JR, Li R, Magnuson JS, Smith RV, Moore E, et al. (2015) Oncologic outcomes after transoral robotic surgery: A multi-institutional study. JAMA Otolaryngol Head Neck Surg 141: 1043-51.
- Lörincz BB, Jowett N, Knecht R (2016) Decision management in transoral robotic surgery: Indications, individual patient selection, and role in the multidisciplinary treatment for head and neck cancer from a European perspective. Head Neck 1: 2190-2196.
- Yeh DH, Tam S, Fung K, MacNeil SD, Yoo J, et al. (2015)Transoral robotic surgery vs. radiotherapy for management of oropharyngealsquamous cell carcinoma - a systematic review of the literature. Eur J Surg Oncol 41: 1603-1614.
- Mandal R, Duvvuri U, Ferris RL, Kaffenberger TM, Choby GW, et al. (2016) Analysis of post-transoral robotic-assisted surgery hemorrhage: Frequency, outcomes, and prevention. Head Neck 1: 776-782.
- Lorincz BB, Mockelmann N, Busch C-J, Knecht R (2014) Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: Single-institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck Nov 37: 1618-1624.
- Setton J, Lee NY, Riaz N, Huang SH, Waldron J, et al. (2015) A multi-institution pooled analysis of gastrostomy tube dependence in patients with oropharyngeal cancer treated with definitive intensity-modulated radiotherapy. Cancer 121: 294-301.
- Dziegielewski PT, Teknos TN, Durmus K, et al. (2013) Transoral robotic surgery for oropharyngeal cancer. JAMA Otolaryngol Neck Surg 139: 1099.
- Chen AM, Daly ME, Luu Q, Donald PJ, Farwell DG (2015) Comparison of functional outcomes and quality of life between transoral surgery and definitive chemoradiotherapy for oropharyngeal cancer. Head Neck 37: 381-385.
- More YI, Tsue TT, Girod DA, Harbison J, Sykes KJ, et al. (2013) Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. JAMA Otolaryngol Neck Surg 139: 43-48.
- Broglie MA, Soltermann A, Haile SR, Röösli C, Huber GF, et al. (2013) Quality of life of oropharyngeal cancer patients with respect to treatment strategy and p16- positivity. Laryngoscope 123: 164-170.
Citation: Viros Porcuna D., Granell J., Rama Lopez J., Pollan Guisasola C., TilPerez G., et al. (2019) Transoral Robotic Surgery for Squamous Cell Carcinoma of the Oropharynx. Outcomes of the First Multicentric Series in Spain. J Otolaryng Head Neck Surg 5: 030
Copyright: © 2019 David Virós Porcuna, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
