
Treating Female Diffuse Hair Loss using Nourkrin® Woman (with Marilex®) - An Open-label, Subjective, Outcome Study on Hair Growth and Appearance, Self-Confidence and Treatment Satisfaction
*Corresponding Author(s):
Erling ThomEtc Research And Development, Oslo, Norway
Tel:+47 91710137,
Email:erlingthom@etc.as
Abstract
Diffuse hair loss in women is a common and significant health issue that negatively affects their body image, self-confidence and perceived quality of life. This condition is still poorly understood by the public and healthcare professionals, and the available therapeutic options are limited. Oral administration of certain specific proteoglycans, known as Proteoglycan Replacement Therapy (PRT), is a novel approach to hair loss with positive outcomes from clinical trials. The present study aimed to evaluate the patients’ perception of the effects of monotherapy with Nourkrin® Woman, a PRT containing a specific complex of bioactive proteoglycans (Marilex®).
Methods
114 women (mean age = 42.9 years) with variable degrees of non-inflammatory diffuse hair loss (female pattern hair loss or telogen effluvium) participated in an open-label, subjective study carried out in the United Kingdom under dermatological supervision from the World Hair Council. Participants voluntarily started a 6-month course of treatment with Nourkrin® Woman (600mg Marilex® per day) and were interviewed every three months using a yes-no questionnaire.
Results
At month three, a significant 92.11% of subjects experienced improvement in the growth of their hair; 93.86% believed their hair had better quality and appearance; 77.19% had an enhanced self-confidence and overall satisfaction with their hair. All participants expressed their overall satisfaction with the Nourkrin® treatment. Satisfaction rates increased when continuing the treatment for six months to 96.49% for hair growth, 97.37% for hair appearance and 80.70% for hair self-confidence. At the end of the study, 98.25% of women with hair loss expressed their overall satisfaction with PRT with Nourkrin® Woman. No significant, treatment-related side effect was reported.
Conclusion
Findings from this investigation support the conclusion that oral PRT with Nourkrin® enhances hair growth and appearance, improves self-confidence and hair satisfaction as perceived by the patients with diffuse hair loss. Overall treatment satisfaction with Nourkrin® has been markedly high in this study.
Keywords
ABBREVIATIONS
AA: Alopecia Areata
FPHL: Female Pattern Hair Loss
KAP: Kingsley Alopecia Profile
MPHL: Male Pattern Hair Loss
PFA: Proteoglycan Follicular Atrophy
PRT: Proteoglycan Replacement Therapy
TE: Telogen Effluvium
INTRODUCTION
Hair is an inseparable part of a woman’s body image and femininity so that it is known as her “crowning glory”. Compared to men, women are much more invested in their hair and are naturally more sensitive to its imperfections. This has been reflected in a survey performed in 1993, where more than half of the female participants believed that “if my hair isn’t right, nothing else can make me feel that I look good” [6]. It thus comes as no surprise that hair loss leaves a devastating impact on a person’s quality of life at a level comparable to serious skin disorders such as severe psoriasis [7]. Even beyond this, the mere preoccupation or fear of losing hair is enough in some individuals to cause clinical mood and anxiety disorders. Specifically, a certain type of body dysmorphic disorder, known as ‘hair loss dysmorphic disorder’, has exclusively been described in persons preoccupied with pathological hair loss [8].
Hair loss in women is frequently presented by a distinct set of causes and symptoms which, in general, define it as a separate entity that demands special approach considerations. An important element being the reality that hair loss in a woman is still not socially accepted and well understood. Even among healthcare professionals, many still do not have a detailed knowledge of this condition which was recognised several decades ago [9]. These factors add to the devastation and stress of patients and disrupt the delivery of proper health care to women with hair loss [5]. From a pathogenetic point of view, evidence indicates that dihydrotestosterone is far less important in causing FPHL than Male Pattern Hair Loss (MPHL). This may be due to a lower expression of androgen receptors and 5-α reductase in hair follicles of women [10]. With regard to TE, some sub types such as postpartum TE are exclusively female while others are more commonly seen in women, e.g. chronic TE [11].
Currently, available therapies for common types of female hair loss are limited. For instance, topical minoxidil is the only available medical option for FPHL. It is supported by moderate-to-low quality evidence. Anti-androgen agents, including finasteride, have been judged to be no more effective than placebo [12]. Beyond that, no targeted medication has been approved for the treatment of TE in women. Unsurprisingly, standard treatment of diffuse hair loss in women is commonly unsatisfactory; a large number of patients are actively looking for an alternative effective treatment.
Proteoglycan Replacement Therapy (PRT) by oral administration of certain marine-sourced proteoglycans is a novel approach that addresses several downstream pathological mechanisms shared between common types of hair loss in women. As shown by in-vivo experiments [13,14], certain proteoglycans play substantial roles in the development and growth cycle of hair follicles. In particular, a proactive pattern of proteoglycan redistribution throughout a follicular cycle suggests their functional involvement in both induction and regulation of cellular activities within the hair follicle [15]. Hence, a disrupted metabolism of key proteoglycans, especially within the dermal papilla, will affect the normal cycling behaviour of hair follicles [16].
Dysmetabolism of proteoglycans happens during follicular miniaturisation and hair thinning. It is presented as a conspicuous shrinkage of proteoglycan-rich parts of the hair follicle. This degenerative pathological phenomenon, known as Proteoglycan Follicular Atrophy (PFA), is understood to be an important pathology in common types of diffuse hair loss. Mitigating PFA by PRT with a specific, proprietary combination of proteoglycans (Marilex®), marketed as Nourkrin®, has shown efficacy in improving hair growth, hair density and reducing miniaturisation in several clinical trials [17-19]. However, the subjective impression of patients about these clinical improvements in different populations has not been sufficiently investigated. The present study has been conducted to elucidate the self-perception of female patients with diffuse hair loss of the efficacy of PRT with Nourkrin® Woman and evaluate their level of treatment satisfaction.
MATERIALS AND METHODS
Study Participants
Our primary exclusion criteria included concomitant use of other hair loss medications, supplements or laser treatments, undergoing hair transplantation or other major surgical procedures involving the scalp, use of medications known to affect the hair growth cycle (e.g. contraceptive pills, anabolic steroids, immunomodulators and cytotoxic or cytostatic drugs) within the previous six months, or having the clinical manifestations of a type of inflammatory alopecia or another clinically-significant, dermatological condition. Pregnancy and breastfeeding were also considered exclusion criteria although no side effects have been reported for PRT in pregnant women. Subjects with a known allergy to fish or shellfish were not included as Nourkrin® Woman contains fish-derived compounds.
Study design
At each follow-up time point, subjects were provided with a structured, self-administered questionnaire including 2-point scale (yes or no) questions to assess their perception of the changes that occurred in their hair growth, quality and appearance over time and the effect of treatment on their self-confidence about their hair. Participants were also asked about their overall satisfaction with the treatment. Questions about the safety and tolerability of Nourkrin® Woman were included in the questionnaires. The World Hair Council (https://worldhaircouncil.com) has independently monitored and supervised all stages of patient enrolment and evaluation throughout this study. This organisation is a non-commercial network of trichologists, dermatologists and hair loss specialists dedicated to improving the lives of people living with hair growth disorders.
Statistical analysis
RESULTS
|
Number of participants |
114 |
|
Age (years), mean (range) |
42.9 (18-64) |
|
Grade of hair thinning/loss (number) |
|
|
1 (Mild) |
34 |
|
2 (Moderate) |
72 |
|
3 (Severe) |
8 |
|
Duration of hair thinning/loss (months), mean(range) |
12.2 (3-20) |
|
History of previous therapy (number) |
|
|
Yes |
60 |
|
No |
54 |
Overall response rates obtained at both follow-up time points are graphically demonstrated in the Figure 1 below. As illustrated, after three months of treatment, 92% of subjects perceived that the growth of their hair increased; 94% recognised a positive change in the appearance and quality of their hair; 77% felt more self-confident and satisfied with their hair. All participants expressed their overall satisfaction with the Nourkrin® treatment. Continuing the therapy for another three months further improved the results in all three subscales and provided an overall treatment satisfaction rate of more than 98% (Figure 1).
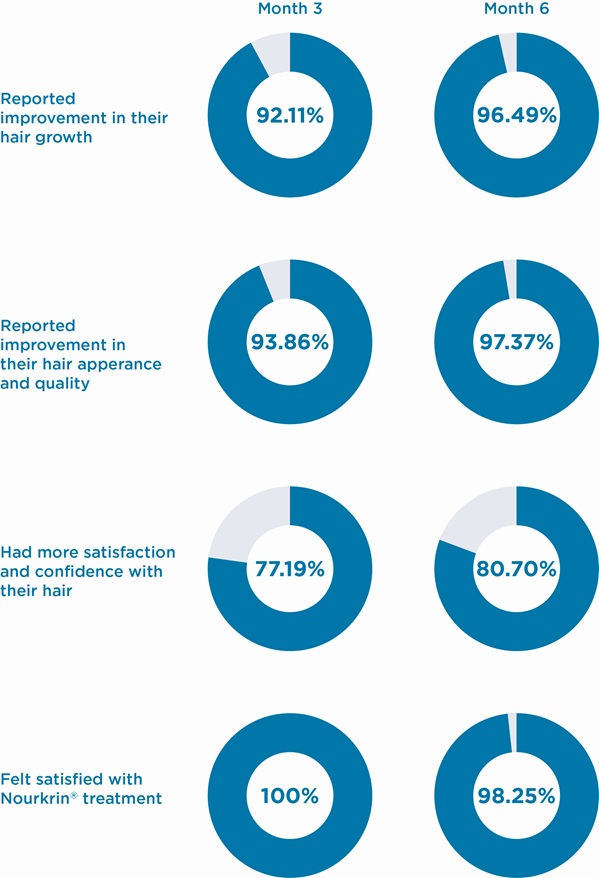
Detailed proportions of responses to all four study questions are presented in Table 2. Based on the results of statistical analyses, self-perceived effects of monotherapy with Nourkrin® Woman on hair growth, appearance/quality and self-confidence have been highly significant at both three- and six-month evaluations with considerable overall treatment satisfaction rates (Table 2).
|
Month 3 |
Month 6 |
|||||
|
Yes |
No |
P-value |
Yes |
No |
P-value |
|
|
Experienced improvement in their hair growth |
105 |
9 |
<0.001 |
110 |
4 |
<0.001 |
|
Experienced improvement in their hair appearance and quality |
107 |
7 |
<0.001 |
111 |
3 |
<0.001 |
|
Had more satisfaction and confidence with their hair |
88 |
26 |
<0.001 |
92 |
22 |
<0.001 |
|
Felt satisfied with Nourkrin® treatment |
114 |
0 |
<0.001 |
112 |
2 |
<0.001 |
In summary, we have observed that monotherapy with Nourkrin® Woman can improve patients’ perception of their hair growth and appearance and, in parallel, elevate their body-image and confidence level. In this longitudinal cohort study, most of the participants (>98%) were satisfied with the outcomes of Nourkrin® treatment at both 3- and 6-month follow-up evaluations. All participants started the treatment voluntarily and all phases of this study have been under close dermatological supervision by the World Hair Council.
During the course of the study, seven minor adverse events in seven subjects were reported; however, none could be attributed to the treatment under study with certainty. These adverse effects were primarily of gastrointestinal nature and disappeared after continuous use. No drop-outs occurred due to adverse events.
DISCUSSION
According to large-scale subjective studies, illness self-perception plays an important role in hair loss patients and is associated with psychological distress and low quality of life [20]. In reality, dermatological assessment of the severity of hair loss does not reliably predict the patient’s perception and the impact of hair loss on an individual’s quality of life [21]. Therefore, the actual success of a therapeutic regimen largely depends on its influence on the patients’ subjective perception of positive changes and their overall treatment satisfaction. This perspective clarifies the significance and need for subjective outcome studies in connection with commonly-used therapeutics in the field of dermatology.
In this study, we aimed to investigate how clinical improvements by Nourkrin®, observed in previous clinical trials, are subjectively perceived by patients and specify treatment satisfaction in a randomly-selected population of women under treatment with Nourkrin®. We used a short and easy-to-complete questionnaire to facilitate participation and maximise participant retention. Our observations indicate that women with diffuse hair loss (female pattern hair loss or telogen effluvium) started to perceive the favourable therapeutic effects of Nourkrin® as early as three months into the treatment. At this point, more than 92% of our subjects felt that the growth and appearance of their hair had improved. Such a uniquely-fast therapeutic effect, so-called the ‘immediate impact’, is due to the an agen-prolongation and -inducing effects of certain proteoglycans in Nourkrin® [13,22-24] which prevent active follicles from entering catagen and force dormant telogen follicles into regrowth. In comparison, a trial in topical minoxidil plus a botanical remedy on women with FPHL recorded a 79% and 88% positive effect on hair volume and appearance, respectively, only after four months of treatment [25]. Importantly, Nourkrin® has expressed the extra advantage of improving the self-confidence level of the majority of patients.
It appears sensible to assume that significant progress in hair growth and quality can lead to discernible improvements in self-perceived quality-of-life in patients with chronic hair loss. This hypothesis has been corroborated by a clinical study designed to examine the subjective effects of therapy with Nourkrin® [17]. A 6-month course of PRT significantly improved the feelings of anxiety and depression, self-confidence, social and sexual activities and work performance in female volunteers with hair loss as measured by the Kingsley Alopecia Profile (KAP). Moreover, authors reported a substantial 39% increase in the overall KAP score for self-perceived quality of life at the end of the study [17], which is comparable with a 50% improvement in the quality of life of female participants after 12 months of treatment with the first-line medication, topical minoxidil, measured by dermatology life quality index [26].
Patient satisfaction, particularly in the management of chronic disorders, is an ultimate goal of treatment and is correlated with the safety and efficacy of a therapy. It may as well influence the actual treatment outcomes [27]. A significantly high satisfaction rate with Nourkrin® monotherapy in this study was observed at both 3- and 6-month evaluations and points to a desirable efficacy and absence of substantive side effects. Similar levels of treatment satisfaction with Nourkrin® have also been described in previous clinical trials on hair loss patients [18,19]. In comparison, the satisfaction rates with topical minoxidil plus a botanical remedy for four months have been reported to be lower (86%) among women [26] and men (28% after six months) with no meaningful changes after three months [28].The lower satisfaction rates with topical minoxidil may have been rooted from its delayed action or common side effects such as dizziness and facial/generalised hypertrichosis [29].On the contrary, the safety and tolerability of Nourkrin® Woman have been confirmed by this study and several previously published clinical trials [17-19].
The results of this study need to be interpreted in the light of a number of limitations; the most important being the absence of a control group and the open-label design of this cohort. Furthermore, the sampling population of the current research has been restricted to the UK. Furthermore, including a range of subjective assessments of hair growth and density could expand the capacity of this study to identify associations between patients’ self-assessment rates and quantitative clinical measurements. The significant findings of this study lay the foundation for future more inclusive research around the effects of Nourkrin® on hair growth and psychological parameters of women with diffuse hair loss.
CONCLUSION
ACKNOWLEDGMENT
REFERENCES
- Scheinfeld N (2008) A review of hormonal therapy for female pattern (androgenic) alopecia. Dermatol Online J 14:1.
- Shrivastava SB (2009) Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venereol Leprol 75: 20-27.
- Camacho-Martínez FM (2009) Hair loss in women. Semin Cutan Med Surg 28: 19-32.
- Davis DS, Callender VD (2018) Review of quality of life studies in women with alopecia. Int J Womens Dermatol 4: 18-22.
- Cash TF (2001) The psychology of hair loss and its implications for patient care. Clin Dermatol 19: 161-166.
- Etcoff N (1999) Survival of the prettiest: The science of beauty (1st edn), Doubleday Inc., New York, USA.
- Williamson D, Gonzalez M, Finlay AY (2001) The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol 15: 137-139.
- Radmanesh M, Shafiei S, Mortazavi ME (2002) Hair Loss Dysmorphic Disorder – a Frequently Encountered and Often Neglected Disorder. Dermatol Psychosom 3: 193-195.
- Ludwig E (1977) Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 97: 247-254.
- Sawaya ME, Price VH (1997) Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 109: 296-300.
- Whiting DA (1996) Chronic telogen effluvium: increased scalp hair shedding in middle-aged women. J Am Acad Dermatol 35: 899-906.
- van Zuuren EJ, Fedorowicz Z, Schoones J (2016) Interventions for female pattern hair loss. Cochrane Database Syst Rev Pg no: CD007628.
- Inui S, Itami S (2014) A newly discovered linkage between proteoglycans and hair biology: decorin acts as an anagen inducer. Exp Dermatol 23: 547-548.
- Couchman JR, McCarthy KJ, Woods A (1991) Proteoglycans and glycoproteins in hair follicle development and cycling. Ann N Y Acad Sci 642:243-51.
- Malgouries S, Thibaut S, Bernard BA (2008) Proteoglycan expression patterns in human hair follicle. Br J Dermatol158: 234-242.
- Elliott K, Stephenson TJ, Messenger AG (1999) Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol 113: 873-877.
- Kingsley DH, Thom E (2012) Cosmetic hair treatments improve quality of life in women with female pattern hair loss. J Appl Cosmetol 30: 49-59.
- Thom E (2006) Nourkrin®: Objective and subjective effects and tolerability in persons with hair loss. J Int Med Res 34:514-519.
- Thom E (2001) Efficacy and tolerability of Hairgain in individuals with hair loss: a placebo-controlled, double-blind study. J Int Med Res 29: 2-6.
- Yu NL, Tan H, Song ZQ, Yang XC (2016) Illness perception in patients with androgenetic alopecia and alopecia areata in China. J Psychosom Res 86:1-6.
- Reid EE, Haley AC, Borovicka JH, Rademaker A, West DP, et al. (2012) Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol 66: 97-102.
- Jing J, Wu XJ, Li Yl, Cai SQ, Zheng M, et al. (2014) Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exp Dermatol 23: 486-491.
- Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, et al. (1999) Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA 96: 7336-7341.
- du Cross DL, LeBaron RG, Couchman JR (1995) Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol 105: 426-431.
- McMichael A, Pham H, von Grote E, Meckfessel MH (2016) Efficacy and Safety of Minoxidil 2% Solution in Combination with a Botanical Hair Solution in Women with Female Pattern Hair Loss/Androgenic Alopecia. J Drugs Dermatol 15: 398-404.
- Zhuang XS, Zheng YY, Xu JJ, Fan WX (2013) Quality of life in women with female pattern hair loss and the impact of topical minoxidil treatment on quality of life in these patients. Exp Ther Med 6: 542-546.
- Dubina MI, O'Neill JL, Feldman SR (2009) Effect of patient satisfaction on outcomes of care. Expert Rev Pharmacoecon Outcomes Res 9: 393-395.
- Faghihi G, Iraji F, Rajaee Harandi M, Nilforoushzadeh MA, Askari G (2013) Comparison of the efficacy of topical minoxidil 5% and adenosine 0.75% solutions on male androgenetic alopecia and measuring patient satisfaction rate. Acta Dermatovenerol Croat 21:155-159.
- Peluso AM, Misciali C, Vincenzi C, Tosti A (1997) Diffuse hypertrichosis during treatment with 5% topical minoxidil. Br J Dermatol 136: 118-20.
Citation: Thom E, Wadstein J (2019) Treating Female Diffuse Hair Loss using Nourkrin® Woman (with Marilex®) - An Open-label, Subjective, Outcome Study on Hair Growth and Appearance, Self-Confidence and Treatment Satisfaction. J Clin Dermatol Ther 5: 037.
Copyright: © 2019 Jan Wadstein, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

