
Treatment of Endometriosis-Related Chronic Pelvic Pain with Ulipristal Acetate and Associated Endometrial Changes
*Corresponding Author(s):
Leah Hawkins BresslerDepartment Of Obstetrics And Gynaecology, Feinberg School Of Medicine, Northwestern University, Chicago, United States
Tel:+1 (919) 966-5283,
Email:Leah_Bressler@med.unc.edu
Abstract
Case: A 25-year-old nulligravidae with endometriosis-related pelvic pain refractory to medical and surgical intervention was administered 15mg ulipristal every other day for 3 months. Daily pain scores and bleeding diary were recorded and serum chemistries and hormone levels were checked prior to, during and after treatment. Pre-treatment and surveillance endometrial biopsy specimens were examined for histology and stained for estrogen and progesterone receptor status. During therapy, pain scores decreased to a median of 0 (P<0.05) and the patient became amenorrheic. Surveillance endometrial biopsy demonstrated SPRM-associated endometrial changes that appeared strikingly similar to simple hyperplasia and resolved with ulipristal discontinuation. Immunohistochemical evaluation demonstrated the presence of estrogen and progesterone receptors before and during ulipristal treatment.
Conclusion: Progesterone receptor modulation with ulipristal substantially improved pain symptoms in a patient with treatment-refractory endometriosis. SPRM-associated changes in the endometrium closely mimicked hyperplasia, developed after less than three months of treatment and resolved after discontinuation of ulipristal and induction of withdrawal bleed.
Keywords
INTRODUCTION
Ulipristal Acetate (ulipristal) is a Selective Progesterone Receptor Modulator (SPRM) that acts as an antagonist and partial agonist, preventing progesterone from binding its receptor. Recently, a large phase II trial of ulipristal for leiomyoma symptom reduction showed that a daily dose taken for 3 months appeared safe and was associated with improved bleeding profiles [3]. Another randomized controlled trial demonstrated that women tolerated daily doses of 10, 20 and 50 mg ulipristal without adverse effects [4]. Considering research demonstrating aberrant progesterone signaling represents a shared common pathway in uterine fibroids and endometriosis [2,5], we hypothesized that progesterone receptor modulation by ulipristal would decrease pain associated with endometriosis. The study was approved by the Northwestern University’s Institutional Review Board and registered with clinicaltrials.gov (Clinical trials #NCT02213081). Our patient provided informed consent prior to trial entry.
CASE DESCRIPTION
Pretreatment endometrial biopsy and labs were within normal limits aside from a mild elevation in alkaline phosphatase which was evaluated by hepatology and determined to be incidental and benign. Prior to treatment, the patient reported daily pain and bleeding scores for 20 days (Table 1, Figure 1) after discontinuing OCPs. Her pretreatment maximum daily pain score was 10 and she used 2-3 pads per day during her menstrual cycle. She was not sexually active. Transvaginal ultrasound was performed before beginning ulipristal acetate and demonstrated a normal-sized, anteverted uterus with no adnexal masses.
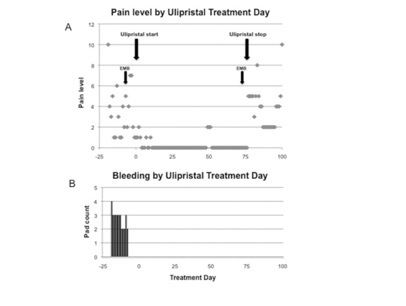 Figure 1: Patient-reported daily pain levels (A) and vaginal bleeding (B) before and during ulipristal treatment.
Figure 1: Patient-reported daily pain levels (A) and vaginal bleeding (B) before and during ulipristal treatment.|
Time period |
Observations (n) |
Mean |
Median |
Range |
SEM |
|
Prior to start of therapy (S) |
20 |
3.5 |
3 |
(1, 10) |
0.57 |
|
Duration of therapy (D) |
74 |
0.2 |
0 |
(0, 2) |
0.06 |
|
Follow-up (F) |
23 |
3.9 |
4 |
(2, 10) |
0.43 |
|
S-Da |
20 |
3.5 |
3 |
(1, 10) |
0.57 |
|
D-Fb |
23 |
-3.9c |
-4c |
(-2, -10)c |
0.43 |
|
S-Fd |
20 |
0.0 |
0c |
(-7, 5)c |
0.72 |
Table 1: Pain scores during ulipristal acetate therapy.
A. Difference in pain scores was significant using Wilcoxon signed rank test with a p-value of 0.00008 (P<0.05).
B. Difference in pain scores was significant using Wilcoxon signed rank test with a p-value of 0.000027 (P<0.05).
C. Negative values depict that pain levels increased after cessation of therapy.
D. Difference in pain scores was not significant using Wilcoxon signed rank test with a p-value of 0.96 (P>0.05).
Within days of therapy, she became amenorrheic (Figure 1). Her pain scores decreased to a median of 0 and were significantly lower than pre-treatment scores (P<0.001, Figure 1). Her maximum pain score during therapy was 2 compared to pre and post-therapy scores of 10. Surveillance labs and hormone (progesterone, estradiol and FSH) levels remained within normal limits. Our patient did not report adverse effects of treatment. Following discontinuation of ulipristal, her median pain level increased to 5 and bleeding increased to 3 pads per day.
Surveillance endometrial biopsy was performed during the third month of treatment, when her endometrial stripe measured 16mm, and demonstrated changes consistent with simple hyperplasia. Correspondingly, ulipristal therapy was immediately discontinued and further enrollment in this planned ongoing clinical trial was halted. Withdrawal bleed was induced with combination OCPs and she was started on daily 5mg norethindrone acetate for 5 days prior to repeat biopsy, which demonstrated normal endometrium (Figure 2). However, in the interim our team requested further pathologic review and after departmental review her endometrial pathology was interpreted as being most consistent with Progesterone Receptor Modulator-Associated Endometrial Changes (PAECs).
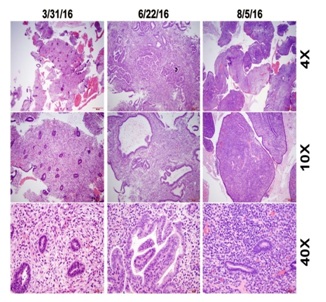 Figure 2: Histologic changes in endometrial biopsies before and after ulipristal treatment.
Figure 2: Histologic changes in endometrial biopsies before and after ulipristal treatment.
All endometrial biopsy specimens underwent immunohistochemical evaluation using estrogen and progesterone receptor antibodies. After deparaffinization and antigen retrieval, immunohistochemical staining was performed on a Ventana Nexus automated system (Tucson, Arizona).
Pre-treatment biopsy revealed typical early proliferative endometrium with no significant pathologic changes (Figure 2). Surveillance endometrial biopsy during the third month of ulipristal treatment was notable for abundant endometrial tissue demonstrating disorganized endometrium with focal glandular crowding. Cystic dilated endometrial glands of varying sizes and shapes with areas of complex and branching glands were present throughout. Rare mitosis and apoptosis were seen in the absence of obvious cytologic atypia. These findings closely mimicked cystic simple endometrial hyperplasia without atypia but were ultimately considered most consistent with features of PAECs (Figure 2). Endometrial biopsy performed one month after discontinuation of ulipristal showed an asynchronous endometrium with rare and scattered endometrial glands with attenuated cuboidal epithelial cells. The hyperplastic changes seen in the surveillance biopsy were absent (Figure 2).
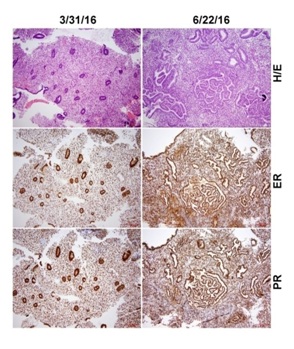
Side-by-side comparison of H&E stained slides with immunostaining for nuclear estrogen and progesterone receptors performed before and during ulipristal treatment did not reveal differences in estrogen and progesterone receptor expression (Figure 3). ER and PR immunostaining showed strong and diffuse positive signals in both endometrial glands and stromal cells in both the biopsy taken before and during treatment (Figure 3).
CONCLUSION
Our finding of improvement in pain scores during ulipristal therapy is consistent with the current understanding of endometriosis pathophysiology. This finding suggests that selective modulation of progesterone receptors impairs endometriosis proliferation and survival. Progesterone resistance in endometriosis may be explained by alterations in progesterone receptors or aberrant expression of progesterone receptor subtypes or of coregulatory proteins. Ulipristal could plausibly disrupt all three pathways. It is possible the observed improvement in pain represents a placebo effect, though this patient’s amenorrhea and concomitant endometrial changes suggest a true effect.
Ulipristal has a specific pharmacodynamic action on the endometrium whereby changes in endometrial thickness, referred to as PAECs, have been demonstrated in animal models [6]. While the development of hyperplasia is rare in women taking ulipristal and the development of PAECs is common, it can be difficult to distinguish the two. In the present case, confusion regarding the interpretation of the surveillance biopsy as simple hyperplasia vs. PAECs highlights a potential diagnostic dilemma that could arise as ulipristal use increases.
In the Pearl II study, the incidence of PAECs among 300 women after 13 weeks of continuous ulipristal therapy was 57-62%; no patients developed hyperplasia [3]. One woman taking ulipristal for 5 years also failed to develop endometrial hyperplasia or PAECs [7]. In our patient, the dosing regimen of 15mg four days a week may have been a factor in the development of PAECs in our patient after only 11 weeks of treatment. Alternatively, it’s possible that BMI impacts the risk of developing PAECs with ulipristal; our patient’s BMI of 42 was higher than the average BMI of 25 in European trials [3].
It was interesting that both estrogen and especially progesterone receptor levels were readily detectable in endometrial biopsy specimens during ulipristal treatment. We speculate that both historically and in this patient, stromal progesterone receptors play an important role in mediating ulipristal action on the epithelial cell [5]. This is supported by existing literature demonstrating that ulipristal action may be mediated primarily by stromal progesterone receptors [2]. Our working theory is that blood vessels carry ulipristal to the tissue, where it first interacts with stromal progesterone receptors.
This case report demonstrates substantial improvement in endometriosis-related pain with progesterone receptor modulation with ulipristal. PAECs were seen with 11 weeks of ulipristal treatment but resolved after discontinuation and withdrawal bleed. Reproduction of results in a prospective investigation would validate the association of ulipristal with endometriosis-related pain improvement presented herein.
ACKNOWLEDGEMENT
REFERENCES
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, et al. (2006) Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248: 94-103.
- Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, et al. (2010) 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med 28: 44-50.
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, et al. (2012) Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366: 409-420.
- Pohl O, Osterloh I, Gotteland JP (2013) Ulipristal acetate - safety and pharmacokinetics following multiple doses of 10-50 mg per day. J Clin Pharm Ther 38: 314-320.
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, et al. (2010) Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151: 2433-2442.
- Pohl O, Williams AR, Bergeron C, Gotteland JP (2013) A 39-week oral toxicity study of ulipristal acetate in cynomolgus monkeys. Regul Toxicol Pharmacol 66: 6-12.
- Levy G, Elkas J, Armstrong AY, Nieman LK (2016) Endometrial Effects of Prolonged Therapy with the Selective Progesterone Receptor Modulator Ulipristal Acetate: A Case Report. J Reprod Med 61: 159-162.
Citation: Hawkins Bressler L, Bernardi LA, Snyder MA, Wei JJ, Bulun S (2017) Treatment of Endometriosis-Related Chronic Pelvic Pain with Ulipristal Acetate and Associated Endometrial Changes. J Reprod Med Gynecol Obstet 2: 008.
Copyright: © 2017 Leah Hawkins Bressler, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

