
Triticum Vulgare Extract in Spray Formulation for Treatment of Vascular Ulcers: A Case Series
*Corresponding Author(s):
Liguori MDepartment Of Angiology, Salvator Mundi Clinic. Rome, Italy
Tel:+39 06588961,
Email:livliguori@tiscali.it
Abstract
Introduction: Vascular ulcers represent a significant health problem due to the economic commitment that long-lasting treatments with advanced dressings require. The long healing time and the high rate of relapse not only worsen the quality of life of the patients but also increase their morbidity and mortality. There are several advanced medications available on the market, including hydrocolloids, foams, hydrogels, spray, ointments and simple non-adherent dressings, without evident differences in efficacy. Among the products used both in hospital and outpatient angiology there is the extract of Triticum vulgare (TVE, the common wheat plant), as the active ingredient of a dressing in different formulations: cream, soaked gauzes, hydrogel or spray. This medical device is proposed in the treatment of skin ulcers due to its well-known ability to stimulate chemotaxis and maturation of fibroblasts thus favoring ulcer Re-epithelization. For this reason, this study was aimed to describe the efficacy and tolerability of the aqueous extract of Triticum vulgare associated with Phenoxyethanol conveyed in the spray formulation (Fitostimoline® Spray, Farmaceutici Damor S.p.A., Italy, based on Rigenase® (Triticum vulgare extract,TVE), Carbomer, Phenoxyethanol, Sodium hydroxide, Purified water) in the treatment of ulcers of different etiology: chronic venous disease, post-thrombotic syndrome, diabetic, arterial and mixed ulcers.
Patients: We selected from the data base of the outpatient clinic of the Division of Angiology of the S. Giovanni Addolorata Hospital in Rome 52 consecutive patients, from January to December 2019, who were treated with TVE in spray formulation for 3 months (with dressing intervals of 3 days and eventually associated with elastic compression bandage). Outcomes were: percentage of complete healing, healing time, clinical features of ulcers as a response to treatment, reduction of pain, assessed with the Visual-Analog Scale (VAS), as well as patient compliance to the dressing. A three-month follow-up was performed at the end of the pharmacological treatment. All patients gave their informed written consent to the use of their clinical data for scientific purposes.
Results: Ulcers healed completely in 65.3% of treated cases (34 patients). In 9 patients (17.3%) there was a good response on inflammation and exudate without complete healing, associated with a reduction in the VAS scale score of pain. There was only one case of relapse after 2 months.
Conclusion: These results suggest that TVE in spray, is a useful medical device that finds particular indication for the treatment of various kinds and types of ulcers, mainly those with low exudate, for its ability to accelerate the granulation process by favoring Re-epithelization and to control inflammation, two components which play a major role in ulcer healing.
Keywords
Granulation; Inflammation; Lower limbs, Triticum vulgare extract; Vascular ulcers
INTRODUCTION
The prevalence of lower limb ulcers is currently around 1% in the global population, with a peak of 3.6% among older individuals in developed countries [1,2]. It is estimated that 1 to 3 million people are affected in Italy [3]. Since the mean age of the population is constantly increasing all over the world, the prevalence of limb ulcers, which is directly related to age, is progressively increasing. Beyond age, the main risk factors for limb ulcers are obesity, history of previous trophic lesions in the lower limbs, deep and superficial venous thrombosis and chronic venous disease, diabetes mellitus and cardiovascular diseases [4]. Vascular ulcers represent a significant health problem due to the economic commitment that long-lasting treatments with advanced dressings require. The long healing time and the high rate of relapse not only worsen the quality of life of the patients but also increase their morbidity and mortality [5,6]. The Italian SICVE-SIF 2016 Guidelines report a complete healing rate of 50-75% in 4-6 months, while 20% of ulcers remain open after 24 months and 8% even after 5 years [2]. There are several advanced medications available on the market, including hydrocolloids, foams, hydrogels, spray, ointments and simple non-adherent dressings, without evident differences in efficacy [7,8]. Among the products used both in hospital and outpatient angiology there is the extract of Triticum vulgare (TVE, the common wheat plant), as the active ingredient of a dressing in different formulations: cream, soaked gauzes, hydrogel or spray. This medical device is proposed in the treatment of skin ulcers due to its well-known ability to stimulate chemotaxis and maturation of fibroblasts thus favoring ulcer Re-epithelization [9-12]. Recent studies have highlighted other characteristics of the TVE, such as an important anti-inflammatory and anti- metalloproteases 9 (anti-MMP9) action [13,14], and a high antioxidant activity on free radicals, which accumulate in vascular lesions and hamper their repair [15].
AIM OF THE STUDY
This study was aimed at describing the efficacy and tolerability of the Damor aqueous extract of Triticum vulgare (TVE, Rigenase®), conveyed in the spray formulation (Fitostimoline® Spray Farmaceutici Damor S.p.A., Italy, based on Rigenase® (Triticum vulgare extract,TVE), Carbomer, Phenoxyethanol, Sodium hydroxide, Purified water), in tissue repair processes in a large population of consecutive patients with venous stasis, arterial or mixed vascular ulcers, recruited in the period between January and December 2019 in the outpatient clinic of the Division of Angiology of the S. Giovanni Addolorata Hospital in Rome. Outcomes were: healing time, clinical features of ulcers as a response to treatment, the percentage reduction in the size of the lesions, reduction of pain assessed with the Visual-Analog Scale (VAS), as well as patient compliance to the dressing.
PATIENTS
We examined the data base of the outpatient clinic of the Division of Angiology of the S. Giovanni Addolorata Hospital in Rome looking for the records of patients of both sexes, aging from 18 to 90 years, suffering from at least one active trophic lesion in the lower limbs, not exceeding 25 cm2, with clinical and instrumental signs attributable to venous insufficiency or obstructive arterial arteriopathy of the lower limbs, or of a mixed type. Patients with the following characteristics or conditions were excluded: presence of pressure sores; pregnancy or breastfeeding; concomitant neoplastic disease; allergic diathesis (known or presumed) to TVE, concomitant treatment with: opioid analgesics, corticosteroids; antineoplastic or immunosuppressant. Patients with medical conditions which could interfere with wound healing were also excluded.
The clinical record included: basal visit with anamnesis, physical examination, routinely blood and urine test [16], arterial and venous ultrasound examination of the lower limbs [17]. In patients with arterial disease the measurement of the ankle / Arm PressureI (ABI) quantified the severity of peripheral arterial disease [18]. Finally, the need of an elastic-compression bandage to the dressing with spray was also evaluated in patients with Chronic Venous Disease (CVD) without arteriopathy. Furthermore, in order to have a homogeneous population, patients with particularly exudating or secreting lesions underwent a 1-2-week pre-treatment with absorbent dressings before starting the application of the spray.
At the time of record evaluation the patients were categorized according to the following criteria: 1) Clinical characteristics of the ulcers (size, appearance of the fundus, type / degree of exudation, perilesional skin, wound granulation tissue, bleeding, infectious / necrotic state, cleansing) and 2) Pain score, assessed by Visual-Analog VAS scale (straight line of 10 cm, with two ends corresponding to “no pain” and “maximum pain” on which the patient must indicate the intensity of the symptoms). This test had also been used for a correct choice of the analgesic therapy. In patients with suspected ulcer infection, a culture swab with antibiogram had been performed for a possible targeted antibiotic therapy [19]. The application of TVE in spray was carried out at each dressing renewal with intervals of 3 days. The dressing was completed with sterile fatty gauzes, and, in patients with CVD without arteriopathy, a dry elastic or zinc oxide compression bandage was also performed. Before applying the TVE in spray, the lesion was thoroughly cleaned with saline solution, and any necrotic and fibrinous debris was mechanically removed with sterile forceps and gauzes. During the treatment, the patients were visited every 2 weeks, for a maximum observational period of 3 months, to assess the previously reported clinical characteristics of the ulcers and the level of pain in the VAS Scale. At the end of the 3 months of treatment, or after healing, the follow-up of the treated patients included a clinical-instrumental check at 30, 60 and 90 days, in order to record ulcer relapse, if any.
RESULTS
Fifty-two patients, 37 females (71.1%) and 15 males (28.8%, aging from 64 to 88 years, mean age 76 years) were considered (Table 1). The etiology of ulcers was multiple: CVD (N=30, 57.6%); post-thrombotic syndrome (PTS) (N=8, 15.3%); diabetic (N=4, 7.6%); arterial (N=3, 5.7%) and mixed ulcers (N=7,13.4%) (Table 2). The main comorbidities were: arterial hypertension (34 patients, 65.3%); heart disease (11 patients, 21.1%); diabetes mellitus (4 patients, 7.6%); obesity / overweight (6 patients, 11.5%); rheumatoid arthritis (5 patients, 9.6%); vasculitis (3 patients, 5.7%) (Table 3). Eight patients reported a history of previous thrombosis, with Deep Vein Thrombosis (DVT) or Superficial Vein Thrombosis (SVT) (15.3%); 1 patient with arteriopathy had a post-traumatic posterior injury extended to the Achille’s tendon. The average surface of the ulcers was 17 cm2, with an average ulceration time of about 12 weeks.
|
52 patients |
|
37 Females (71.1%) |
|
15 Males (28.8%) |
Table 1: Number of patients.
|
Ulcer Etiology |
|
CVD (57.6%) |
|
PTS (15.3%) |
|
Diabetic (7.6%) |
|
Arterial (5.7%) |
|
Mixed (13.4%) |
Table 2 Ulcer etiology
|
Main Comorbidities |
|
Arterial Hypertension (65.3%) |
|
Heart Disease (21.1%) |
|
Diabetes Mellitus (7.6%) |
|
Obesity/ Overweight (11.5%) |
|
Rheumatoid Arthritis (9.6%) |
|
Vasculitis (5.7%) |
Table 3: Main comorbidities of the patients.
34 out of 52 patients enrolled (65.3%) showed a complete healing of the ulcer at the end of the 3-months of treatment. In 9 patients (17.3%) there was a marked reduction (>40%) in the ulcerated area with improvements in both morphological characteristics of the ulcer and pain. In 7 (13.4%) patients including 3 with CVD ulcer, 2 with mixed genesis (venous stasis and arteriopathy) and 2 diabetics, the ulcer showed no sign of improvement. Worsening of the ulcer, due to the subsequent infection, was observed in 2 patients with mixed genesis ulcer (3.8%), who were hospitalized and one of the two underwent endovascular revascularization (Figure 1).
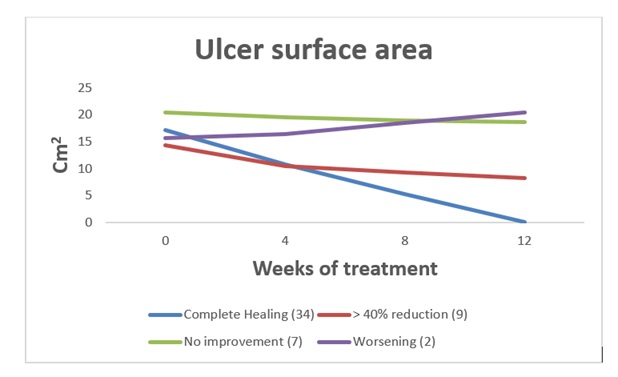 Figure 1: Time course of mean ulcer surface area in 4 different groups of patients.
Figure 1: Time course of mean ulcer surface area in 4 different groups of patients.
In the 34 patients who completely recovered, a rapid tendency to granulation of the bottom of the ulcer, with an area reduction of more than 40% was observed already after the first 5-6 weeks of treatment. The average healing time was 9 weeks. The average size of the ulcer, measured at time 0 in all patients enrolled, was approximately 17 cm2. In the 9 (17.3%) patients with a reduction greater than 40% of the basal value of the ulcerated area, the average area of the ulcer decreased by about 42% compared to time 0 (Figure 2); and there was also a good response both in terms of reduction of exudate and peri-lesional inflammation, as well as granulation of the fundus. Pain was present at enrollment in 40 out of 52 patients and the VAS scale showed an average value of 5.4 (0-10) with a tendency to a higher value for patients with PTS and mixed etiology (arterial-venous) ulcers. In the 9 patients who showed an improvement of the skin lesion without a complete healing VAS had an evident reduction in the average pain value at the end of the quarterly observation, moving from a score of 6.2 to 3.5 (Figure 3). Only 12 patients required NSAIDs for pain relief (8 with PTS and 4 mixed etiology) for short periods, and in 9 an oral targeted antibiotic therapy based on the results of the antibiogram was performed. No patient showed spray intolerance or allergies to TVE. In the follow-up, only 1 patient with post-thrombotic syndrome had relapse of the malleolar ulcer after two months, probably on account of poor compliance with the elastic stocking.
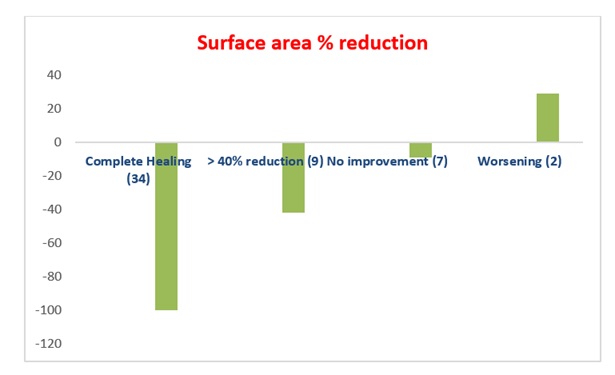 Figure 2: Percentage reduction of the ulcer surface area at the end of the study compared to the time 0 value.
Figure 2: Percentage reduction of the ulcer surface area at the end of the study compared to the time 0 value.
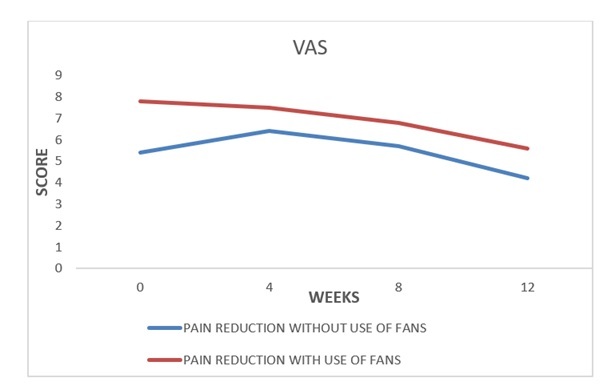 Figure 3: Time course of the mean VAS score in patients treated with TVE alone (n=40 ) or associated with FANS (n=12).
Figure 3: Time course of the mean VAS score in patients treated with TVE alone (n=40 ) or associated with FANS (n=12).
DISCUSSION
The fundamental cornerstones of the treatment of venous ulcer are represented by local care and elastic restraint [1,2]. With regard to local therapy, there is no clear evidence in the literature on the efficacy of the multiple medications available for the different types of vascular ulcers. Elastic compression should instead be recognized for its ability to reduce venous hypertension, to counteract stasis edema, which causes the deposition of insoluble substances in the tissues, to promote healing of the ulcer and prevent recurrence [1,20]. In literature there are criteria of classification and management of ulcers expressed in the “positioning document” of the Italian Association of Cutaneous Ulcers (AIUC), with the concept of Wound Bed Preparation (WBP) to accelerate the endogenous healing process [21]. However, the choice of the treatment is based on the individual physician’s knowledge of the patient and the condition of the ulcer at the time of observation [22]. In diabetic patients, the healing of neurovascular ulcers is essential to avoid gangrene, deep tissue infections or osteomyelitis leading to amputations and disabilities [23].
TVE has a recognized tissue stimulating effect highlighted by various studies [9-12, 24, 25]. Farinella, et al. [9] reported that the TVE extract induces a 72% increase in the growth of 3T3 murine fibroblasts in cell culture as compared to that of the Fibroblast Growth Factors (FGF). This favorable result was due to a significant increase in the activity of the Enzyme Ornithine Decarboxylase (ODC) which potentiates the uptake of proline (an amino acid constituting the collagen chains) by the tissues. Sanguigno, et al. [10], focusing on increased fibroblastic index, chemotaxis and fibroblastic maturation, crucial for the skin repair process, promoted by the TVE, identified the pharmacologically active fractions of the TVE. Fibroblasts are the most numerous cells of the connective tissue and produce extracellular matrix and collagen thus promoting granulation tissue which functions as a support for the subsequent re-epithelialization process. Tito, et al. [11], showed that TVE possessed promising potential to be developed as a wound healing promoting agent in skin care and dermatology.
According to the histological evaluation reported by Mastroianni, et al. [25], cell proliferation is favored by the mitogenic effect exerted by TVE both on fibroblasts and on dermal and epidermal endothelial cells. Recently, important anti-inflammatory and antioxidant properties of the TVE have been highlighted. In particular, Sanguigno, et al. [13] reported that, in a murine microglia inflammation model, TVE reduces the release of all the evaluated inflammation mediators, such as Nitric Oxide (NO), interleuchin-6 (IL6), tumor necrosis factor alpha (TNF alpha) and prostaglandin E2 (PGE2). Moreover, Antonucci, et al. demonstrated a high antioxidant capacity of TVE. The ability of TVE to eliminate the free oxygen radicals that are localized in vascular lesions (reactive oxygen species (ROS): superoxide radical, hydrogen peroxide, hydroxyl radicals) and cause oxidative stress [15], cell degeneration and inflammation, emerged from the test of inhibition of hemolysis and from the ORAC test (of fluorescein oxidation) which showed a radical washing activity of TVE higher than that of ascorbic acid.
The different properties set out show a regenerative, protective and reparative action of the TVE which are evident mainly in poorly exudative ulcers which require a simultaneous antibacterial and tissue granulation stimulatory action.
In conclusion, we documented a high degree of patient satisfaction due to ulcer healing or at least improvement with a significant reduction in perceived pain. This observation confirms the clinical relevance of the lesion induced pain, as is currently underlined by the various Italian and International consensus documents on the management of dressings [26-29]. Patient compliance was optimal with no drop-out cases.
These results support the conclusion that the TVE spray is a useful aid that finds particular indication in the treatment of various kinds and types of ulcers, especially in those with low exudate, for its ability to accelerate the granulation process and to improve the local inflammatory state, main determinants of ulcer healing.
The Authors have no conflict of interest to declare.
REFERENCES
- Agus GB, Allegra C, Arpaia G, De Franciscis S, Gasbarro V (2013) Linee Guida Collegio Italiano di Flebologia CIF Revisione 2013. Acta Phleb Ago 14.
- Ebner H, Stillo F, Lanza G, Mangialardi N, Agus GB, et al. (2016) Linee guida flebo-linfologiche SIF-SICVE 2016 della Società Italiana di Flebologia e della Società Italiana di Chirurgia Vascolare ed Endovascolare. Minerva Cardioang Ago 4: 1-80.
- Apollonio A, Antignani PL, Di Salvo M, Failla G, Guarnera G, et al. (2012) Epidemiologia e cura delle ulcere vascolari in Italia 2012. Preview dello studio SUV (AIUC Settembre 2012). Acta Phleb 13: 101.
- Nelson EA, Bell-Syer SE, Cullum NA (2000) Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev.
- Ruckley CV (1997) Socioeconomic impact of chronic venous insufficienty and leg ulcers. Angiology 48: 67-69.
- MDeC Silva (1991) MChronic venous insufficiency of the lower limbs and its Socio-Economic significance. Int. Angiol 10: 152-157.
- Palfreyman S, Nelson EA, Michaels JA (2007) Dressing for venous leg ulcers: Systematic review and meta-analysis. BMJ 335: 244.
- Warriner RA, Carter MJ (2011) The current state of evidence-based protocols in wound care. Plast Reconstr Surg 127: 144-153.
- Farinella Z, Morale MC, Agosta MA, Rizza V (1986) Stimulation of cell division in mouse fibroblast line 3T3 by an extract derived from Triticum vulgare. Int J Tissue React 8: 337-342.
- Sanguigno L, Minale M, Vannini E, Arato G, Riccio R, et al. (2015) Oligosaccharidic fractions derived from Triticum vulgare extract accelerate tissue repairing processes in in vitro and in vivo models of skin lesions. J Ethnopharmacol. 159: 198-208.
- Tito A, Minale M, Riccio S, Grieco F, Colucci MG, et al. (2020) A Triticum vulgare Extract Exhibits Regenerating Activity During the Wound Healing Process. Clin Cosmet Investig Dermatol 13: 21-30.
- D’Agostino A, Pirozzi AVA, Finamore R, Grieco F, Minale M, et al. (2020) Molecular Mechanisms at the Basis of Pharmaceutical Grade Triticum vulgare Extract Efficacy in Prompting Keratinocytes Healing. Molecules 25: 431.
- Sanguigno L, Casamassa A, Funel N, Minale M, Riccio R, et al. (2018) Triticum vulgare extract exerts an anti-inflammatory action in two in vitro models of inflammation in microglial cells. Plos One 13: 0197493.
- Funel N, Dini V, Janowska A, Loggini B, Minale M, et al. (2020) Triticum vulgare Extract Modulates Protein-Kinase B and Matrix Metalloproteinases 9 Protein Expression in BV-2 Cells: Bioactivity on Inflammatory Pathway Associated with Molecular Mechanism Wound Healing. Mediators Inflamm.
- Antonucci I, Fiorentino G, Contursi P, Minale M, Riccio R, et al. (2018) Antioxidant capacity of Rigenase, a specific aqueous extract of Triticum vulgare Antioxidants (Basel) 7: 67.
- Liang H, Xie Z, Liu B , Song X, Zhao G (2019) A Routine Urine Test Has Partial Predictive Value in Premature Rupture of the Membranes, J Int Med Res 47: 2361-2370.
- Peripheral Arterial Diseases (Diagnosis and Treatment of) Guidelines ESC Clinical Practice Guidelines
- Crawford F, Welch K, Andras A, Chappell FM (2016) Ankle Brachial Index for the Diagnosis of Lower Limb Peripheral Arterial Disease. Cochrane Database Syst Rev 9: CD010680.
- Riccioni C, Sarcinella R, Izzo A, Palermo G, Moschetti B, et al. (2001) About prevention and treatment of venous leg ulcers of the limbs. Our experience. Acta Phleb 1: 13-21.
- Fletcher A, Cullum N, Sheldon TA (1997) A systematic review of compression treatment for venous leg ulcers. BMJ 315: 576-580.
- Paggi B, Dini V, Barbanera S, Macchia M, Panduri S, et al. (2014) La Wound Bed Preparation. Associazione Italiana Ulcere Cutanee. Documento di posizionamento AIUC sulla medicazione dell’ulcera cutanea. AIUC Position Wound Dressing. Acta Vulnologica 3: 111-6.
- Antignani PL (2007) Ulcere vascolari. Ed Minerva Med.
- Masina M (2014) Is there a gold standard in wound management? Acta Vulnologica 3: 153-58.
- Rafanelli A, Saponati G, Rafanelli S (2008) Efficacy and tolerability of Fitostimoline gauze in the epicutaneous treatment of ulcerated dystrophic skin lesions and delayed cicatritial healing. Acta Vulnologica 6: 133-137.
- Mastroianni A, Celleno L, Borgia MG, Cerimele D (1998) L’estratto acquoso di «triticum vulgare». Valutazione clinico-istologica nei processi riparativi tissutali cutanei. Giornale Italiano di Dermatologia e Venerologia 133: 145-53.
- Guarnera G, Bonadeo P, Marchitelli E, Crespi A (2010) Italian Wound Care Association Position Document. Associazione Italiana Ulcere Cutanee (AIUC) Documento di Posizionamento. La terapia farmacologica e chirurgica dell’ulcera venosa. Ulcera venosa, dolore e qualità di vita. Acta Vulnologica 4: 245-249.
- Mosti G (2014) La medicazione dell’ulcera e i dati della letteratura. Documento di posizionamento AIUC (Associazione It. Ulcere Cutanee) sulla medicazione dell’ulcera cutanea. Acta Vulnologica. 3: 117-21.
- EWMA (2002) European Wound Management Association, EWMA Position Document. EWMA.
- WUWHS (2007) World Union of Wound Healing Societies, WUWHS principles of best practice: minimizing pain at dressing-related procedures. A consensus document. Wound Pedia Inc, Toronto, Canada.
Citation: Gallucci M, Liguori M, Boirivant R, Carlizza A, Riccioni C, et al. (2020) Triticum Vulgare Extract in Spray Formulation for Treatment of Vascular Ulcers: A Case Series. J Angiol Vasc Surg 5: 045.
Copyright: © 2020 Gallucci M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

