
Truncular Venous Malformations: Venous Aneurysm Case Series in a General Hospital
*Corresponding Author(s):
Felipe Gerardo Rendón ElíasCentro Universitario De Flebologia Y Malformaciones Vasculares, Servicio De Cirugía Torácica Y Cardiovascular, Hospital Universitario “Dr. José Eleuterio González”, Nuevo León, Mexico
Tel:+ 81 83467800; + 81 834 88305,
Email:drfrendon@gmail.com
Abstract
Primary venous aneurysm corresponding to the truncular venous malformations or to the malformations of major named vessels according to the Hamburg classification and the updated classification from International Society for the Study of Vascular Anomalies respectively. Venous aneurysms are considered an infrequent clinical entity and their natural history and treatment depends of their size and location.
Method
A systematic retrospective analysis of clinical records from patients with the diagnosis of truncular venous malformations type superficial venous aneurysms in the period from September 2014 to December 2017 was reviewed.
Result
A total of 25 patients were identified. The anatomic location of the truncal venous a malformation was neck (7 patients), in the upper extremities (6 patients) and in the lower extremities (12 patients). The patient’s presentation comprised: pain, swelling, thrombosis, aesthetic discomfort and asymptomatic mass. Treatment was surgical in the most of the cases.
Conclusion
Primary venous aneurysms are not rare and they must not be considered just as a varicose vein. The diagnosis is made by a clinical history and physical examination and corroborated with vascular ultrasound and in selected cases other imaging studies are indicated. Surgery is the best therapeutic strategy considering their potential morbidity and mortality.
Keywords
INTRODUCTION
Truncular venous malformations affecting the major named vessel regarding abnormal caliber of the vein can be presented either as stenosis/obstruction or dilatation known as venomegalia (phlebectasia) or aneurysm [5,6], as the outcome of aplasia, hypoplasia, or hyperplasia following defective development during the latter stage of embryogenesis [6,7].
The purpose of this article is to present our experience of patients with superficial truncal venous malformations type aneurysm affecting neck, upper extremities, lower extremities and additionally, the literature is reviewed with a discussion on clinical implications and the diagnostic and therapeutic approach of this lesser known vascular pathology.
PATIENTS AND METHODS
In this article the terminology used to describe venous dilatations were: 1) phlebectasia or venomegalia defined as a diffuse dilatation of one or more veins with a caliber increase ≥ 50% compared with normal [8,9] 2) primary venous aneurysm is defined as a solitary area of venous dilatation that is ≥ 1.5 times the diameter of the normal proximal and distal vein size [10,11], that containing all 3 layers of the vein wall that communicates with a main venous structure by a single channel and must have no association with an arteriovenous communication or pseudoaneurysm, nor be related to a varicose vein ( chronic venous insufficiency) [12]. We just included patients that met the required criteria to classify her venous pathology as a primary venous aneurysm and we reviewed hospital records for patient’s demographic characteristics, clinical presentation, diagnosis approach, treatment and evolution.
RESULTS
Truncular venous malformations in the neck (Table 1)
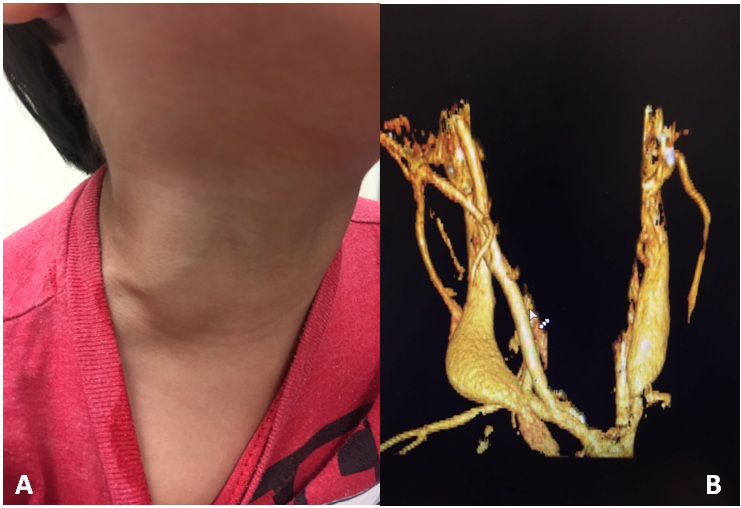 Figure 1: A) 5-year-old patient presented with a lump at the right neck which had been enlarging progressively over a period of a few months. The lesion corresponded to an aneurysm of the external jugular vein. B) Image showing a CAT 3D reconstruction of aneurysmal dilatation at the proximal segment of the right external jugular vein.
Figure 1: A) 5-year-old patient presented with a lump at the right neck which had been enlarging progressively over a period of a few months. The lesion corresponded to an aneurysm of the external jugular vein. B) Image showing a CAT 3D reconstruction of aneurysmal dilatation at the proximal segment of the right external jugular vein.|
Truncal Venous Malformations in the Neck |
||||||
|
|
Sex |
Age |
Site |
Medical history |
Presentation |
Treatment |
|
Case 1 |
M |
3 |
Right External jugular vein |
None |
Asymptomatic nonpulsating mass |
Conservative |
|
Case 2 |
M |
2 |
Right External Jugular Vein |
None |
Asymptomatic nonpulsating mass |
Conservative |
|
Case 3 |
F |
24 |
Left Internal Jugular Vein |
None |
Aesthetic Discomfort |
Surgery |
|
Case 4 |
F |
13 |
Right External Jugular Vein |
None |
Aesthetic Discomfort |
Surgery |
|
Case 5 |
M |
5 |
Right Internal Jugular Vein |
None |
Asymptomatic nonpulsating mass |
Conservative |
|
Case 6 |
M |
3 |
Left External Jugular Vein |
None |
Asymptomatic nonpulsating mass |
Conservative |
|
Case 7 |
M |
5 |
Right External Jugular Vein |
None |
Asymptomatic nonpulsating mass, fear of his parents |
Surgery |
Truncular venous malformations in the upper extremities (Table 2)
 Figure 2: 38-year-old patient presented with painful mass at the right forearm. The lesion corresponded to an aneurysm of the right radial vein external.
Figure 2: 38-year-old patient presented with painful mass at the right forearm. The lesion corresponded to an aneurysm of the right radial vein external.|
Truncal Venous Malformations in the Upper Extremities |
||||||
|
|
Sex |
Age |
Site |
Medical history |
Presentation |
Treatment |
|
Case 8 |
M |
47 |
Left Cephalic Vein |
None |
Pain, Thrombosis |
Surgery |
|
Case 9 |
F |
38 |
Right Radial Vein |
None |
Pain, Thrombosis |
Surgery |
|
Case 10 |
F |
53 |
Right Basilic Vein |
None |
Pain, Thrombosis |
Surgery |
|
Case 11 |
F |
28 |
Right Medial Cubital Vein |
None |
Pain, Thrombosis |
Surgery |
|
Case 12 |
F |
23 |
Right Dorsal Venous Arch |
None |
Non Pulsating Mass, Aesthetic Discomfort |
Surgery |
|
Case 13 |
M |
3 |
Left Basilic Vein |
None |
Pain, Thrombosis |
Surgery |
Truncular venous malformations in the lower extremities (Table 3)
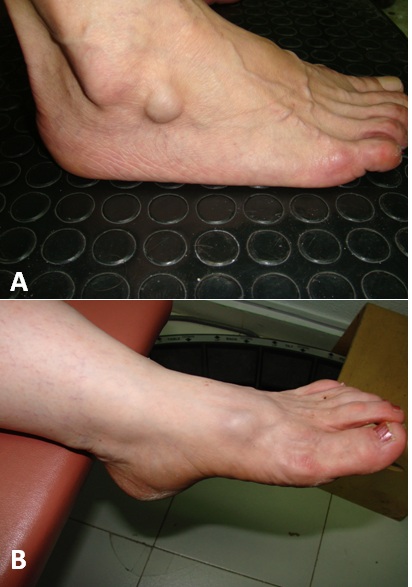 Figure 3: A) 52-year-old patient presented with a compressible mass in the lateral portion of the right venous dorsal foot arch B) 56-year-old patient presented with asymptomatic mass at the left venous dorsal foot arch that cause esthetic discomfort.
Figure 3: A) 52-year-old patient presented with a compressible mass in the lateral portion of the right venous dorsal foot arch B) 56-year-old patient presented with asymptomatic mass at the left venous dorsal foot arch that cause esthetic discomfort.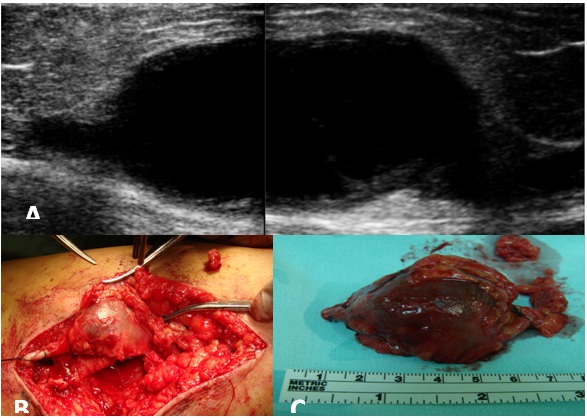 Figure 4: A) Reconstructed ultrasound image which shows an internal saphenous vein venous aneurism of 9 × 5 cm B) Surgical excision of the internal saphenous vein venous aneurisms C) Thrombosed internal saphenous vein venous aneurysm.
Figure 4: A) Reconstructed ultrasound image which shows an internal saphenous vein venous aneurism of 9 × 5 cm B) Surgical excision of the internal saphenous vein venous aneurisms C) Thrombosed internal saphenous vein venous aneurysm.|
Truncal Venous Malformations in Lower Extremities |
||||||
|
|
Sex |
Age |
Site |
Medical history |
Presentation |
Treatment |
|
Case 14 |
F |
62 |
Right GSV* |
None |
Pain, Non Pulsating Mass |
Surgery |
|
Case 15 |
F |
48 |
Right GSV* |
None |
Pain, NonPulsating Mass |
Surgery |
|
Case 16 |
F |
37 |
Right GSV* |
None |
Pain, NonPulsating Mass |
Surgery |
|
Case 17 |
M |
56 |
Right GSV* |
None |
Pain |
Surgery |
|
Case 18 |
M |
61 |
Left GSV* |
None |
Asymptomatic,Non Pulsating Mass |
Surgery |
|
Case 19 |
M |
28 |
Right GSV* |
None |
Asymptomatic non pulsating mass |
Surgery |
|
Case 20 |
F |
53 |
Right GSV** |
None |
Pain, Thrombosis |
Surgery |
|
Case 21 |
M |
48 |
Posterior Arch of GSV |
None |
Asymptomatic |
Surgery |
|
Case 22 |
M |
18 |
Right small saphenous vein |
None |
Acute swelling of the leg |
Surgery |
|
Case 23 |
F |
56 |
Left venous dorsal foot arch |
None |
Asymptomatic, non pulsating mass, aesthetic discomfort |
Surgery |
|
Case 24 |
F |
52 |
Right venous dorsal foot arch |
None |
Pain |
Surgery |
|
Case 25 |
M |
41 |
Right GSV** |
None |
Pain, Non pulsating mass |
Surgery |
DISCUSSION
Vascular anomalies in the past were confused by ambiguous and inconsistent nomenclature, leading to inappropriate treatment and misdirect research efforts. To avoid these problems, two classification systems have been established based on the workshop held in Hamburg, Germany in 1988 [2,13-15] and the other proposed by Mulliken and Glowacki, now adopted by the ISSVA in 1996 (last update in 2018) [1,15-18]. In the Hamburg Classification (HC), accounts for the underlying anatomical, histological, and pathophysiological features of congenital vascular malformations. It also introduces embryological aspects, further subdividing them into either an extratruncular or truncular form, based on the time of developmental arrest during embryonic life. In the ISSVA classification the vascular anomalies are divided in vascular tumors and vascular malformations. Vascular malformations are divided into four groups: simple malformations, combined malformations, malformations of major named vessels which are equivalent to “truncular” type malformation by Hamburg Classification and the malformations associated with other anomalies. Simple malformations are categorized by the type of vessel involved: capillaries, lymphatics, veins or arteries. They affect only one type of vessel, with the exception of arteriovenous malformation that affects arteries, veins and capillaries.
In ISSVA classification the AV belong to the group of the malformations of major named vessels that include malformations that affect veins, and consist of anomalies in the origin, course, number, length, diameter (aplasia, hypoplasia and ectasia/aneurysm) or valves. And in the HC the AV correspond to a venous malformation of the truncular type which can be presented as aplasia, hyperplasia, stenosis (i.e., the left iliac vein in May-Turner syndrome), dilations or aneurysms (the most common being the popliteal vein). For the knowledge of these classifications we decided to name our article like Truncular Venous Malformations: Superficial Venous Aneurysm.
The VA were first mentioned in the medical literature by Harris [19], he described an infant with congenital venous aneurysm of the mediastinum in 1928, and Hilscher [11] suggested the term of venous aneurysm, similar to arterial aneurysms.
More than one in the medical community think that VA are a very rare disorder even some believe that they do not exist and that every dilated veins are varicose vein for this reasons and the lacking of the epidemiologic data make to them a very rare pathology.
Venous aneurysms according to the etiopathogeny can be divided into primary (congenital) and secondary (acquired). The latter type is caused by trauma, by infection or venous valve insufficiency, or by an arteriovenous fistula, which is due to an increase in the venous blood flow [20]. Primary Venous Aneurysms (PVA) are less common but have been reported to occur in most major veins. PVA in the lower extremities (the most common location for VA) also can be classified into superficial AV or deep AV. The incidence of super?cial venous aneurysms is approximately 0.1%, with a prevalence of 1.5%, equally in both sexes and it may occur at any age. In a study by Gillespie et al., [21], 77% of the venous aneurysms were located in the lower extremities (57% of which were in the deep venous system); 10% were located in the upper extremities; and 13% involved the internal jugular vein.
The terminology used to describe venous dilatations can cause confusion. The terms phlebectasia, varicose vein and/or venous aneurysm are considered synonyms in the medical vocabulary; however, they mean different things. Phlebectasia is de?ned as a fusiform and diffusely dilated vein. The association of dilated and tortuous veins is known as varicose veins [22]. There are no precise criteria regarding size to consider a venous dilation like an aneurysm; however, Mateo [12] and McDevitt [23] established that whenever the vein’s diameter is twice as large as the normal diameter, then it is considered to be an aneurysm [24]. Nevertheless, in order to consider it a primary venous aneurysm size is not the only factor considered. It must also be a localized dilation, conformed by three histological layers which constitute the normal venous wall. This could be saccular or fusiform (an important distinction because of its hemodynamic implications that in?uence the course of treatment) and should only communicate to the corresponding vein in a proximal and distal manner and neither be secondary to an arteriovenous ?stula, nor be related to a varicose vein [25]. Abbott was the one who integrated the criteria to de?ne VA [11]. All our patients met the required criteria to classify her venous pathology as a primary venous aneurysm.
The etiology of a PVA is difficult to understand. The much localized nature of these lesions suggests a specific abnormality in the vein wall. The histologic findings in some cases of VA vary from a normal vein wall to marked medial disruption and inflammation. In all these changes of the vein wall the tyrosine kinase receptor TIE2, located on Endothelial Cells (ECs), and its ligand Angiopoietin-1 (ANG-1), secreted by vascular smooth muscle cells (that play a major role in the maturation and stability of veins) may be involved in the origin of the VA [26-30]. The malfunction of the TIE2 could be the guilty factor that provoke the venous remodeling termed endophlebohypertrophy and endo phlebosclerosis that different researches think that they may be important factors in the development of VA. Also in a recent study, tissue from the wall of venous aneurysms was examined and it was reported that structural changes of the aneurysmatic wall can be related to the increased expression of metalloproteinase, which can be translated to a reduction of the muscle layer, fragmented elastic tissue, an increase in the ?brous tissue and in?ltration of in?ammatory cells [31]. Schatz and Fine [32] believed that endophlebohypertrophy was a major factor in the formation of VA, which begins with an increase in venous ?ow leading to a hypertrophy of the venous wall, then dilation and sclerosis. Lev and Saphir [33,34] found that endophlebohypertrophy begins at birth and is associated with areas of stress and endo phlebosclerosis (thinning of the vein wall) increase with age and occurs immediately adjacent to the artery.
Venous aneurysms in the neck
Perhaps the reason why venous aneurysms are rare is due to the low intravascular pressure in the superior vena cava system [37,38]. Jugular Aneurysm (JA) can be saccular or fusiform, the latter is the most common presentation.
Typically, the clinical presentation of the JA is like a unilateral soft mass in the neck, it enlarges on straining, crying, coughing, and Valsalva maneuver. The mass is almost always asymptomatic, although some patients refer the feeling of constriction, sensation of choking and giddiness, bluish discoloration, discomfort during physical activity, swallowing, and cessation of voice during reading or speaking out load [39]. Several characteristics in the clinical examination of a neck mass are suggestive of venous aneurysm. These include easy compressibility, increase in size with Valsalva’s maneuvers and absence of pain. The JA is more commonly on the right internal jugular vein and bilaterality is uncommon.
In the differential diagnosis of a cervical mass, lymphatic malformation, hemangioma, a laryngocele, an enterogenous cyst, thyroid swelling, lymphadenopathy, a thyroglossal cyst, a dermoid cyst and a branchial cleft cyst should be considered. Engorgement of the neck swelling during strain eliminates others than a laryngocele and jugular vein aneurysm [40,41]. The absence of air inside the lesion on plain roentgenography further eliminates the laryngocele [37].
Diagnosis of jugular vein aneurysms can be achieved by color Doppler US imaging (with and without Valsalva), computed tomographic angiography, magnetic resonance angiography imaging, and venography. The Doppler US is the diagnostic method of choice [42].
Because the JA is uncommon treatment guidelines are not established so the management of JA is controversial, especially in asymptomatic cases. Spontaneous rupture has never been described although massive bleeding during tonsillectomy has been report [43]. The risk for thrombotic complications is very low, there is no report about pulmonary embolism and just very few cases of spontaneous thrombosis has been reported [44-47]. For this reasons, most doctors strongly recommended conservative treatment [41,42,48].
Other prefers to follow an aggressive approach with liberal surgical repair [49].
The arguments to choice a surgical treatment are fear of enlargement, fear of rupture, thromboembolic complications and cosmetic and psychologic consideration [50]. It also allows surgeons to get the exact histopathological diagnosis.
The surgical treatment in JA can be safely treated by excision and ligation. Whereas, an exclusion and bypass may be needed in some cases of a fusiform aneurysm to avoid complications (cerebral edema) [49,50].
In our series of Neck vein aneurysm, were most common localized in the right side, all unilateral, and presenting likes an asymptomatic soft mass compressible, changing its size with Valsalva’s maneuvers. We just need a DUS to corroborate the diagnosis; just one patient came with us already with MRI. Before to decide a treatment the patients and the parents were informed about the benign evolutions of these anomalies, but one parents were afraid of the future evolution and the other women because aesthetic discomfort preferred the surgical treatment but careful follow up is an appropriate treatment for primary venous aneurysm in the neck.
Venous aneurysm in the upper extremities
Patients with this condition usually have an asymptomatic subcutaneous mass that sometimes increases gradually in size and may be accompanied by the appearance of new symptoms [53]. The majority of upper extremity VA is initially noted due to aesthetic concerns regarding a soft mass that often enlarges with a Valsalva maneuver [54]. Complications in the upper extremities VA have been recognized, including rupture, venous obstruction and compression of adjacent structures [55,56].
With regard to diagnosis, history and directed physical examination will provide a great deal of information, particularly if attention is paid to positional changes in the morphology of the aneurysm. These maneuvers will help to differentiate venous aneurysms from soft tissue tumors, which have often been reported as the initial diagnosis. Duplex ultrasonography and venography provide significant information regarding the anatomy and physiologic impact of these venous aneurysms. Axial imaging, including both CT and Magnetic Resonance Imaging (MRI), provide additional information regarding the size of the aneurysm along with their relation to surrounding structures [56-58].
The treatment of VA of the upper extremities is less well-defined; based on the limited experience with upper extremity venous aneurysms; it remains unclear whether aggressive surgical management is warranted, particularly for asymptomatic patients, as resection is not without associated morbidity [59]. Since primary superficial VA has no life-threatening complication without thrombus, if the patient has no symptoms or cosmetic demands, regular follow-up may be advised and occurred in the current case. Otherwise, surgical excision can be considered [60].
All our cases in the upper extremities had the characteristics of primary VA, DUS played an important role in the diagnosis, because the most common presentation in our series was thrombosis there was no problem to chosen the surgical treatment to avoid complications, such as pulmonary embolism and one was for cosmetic demand. If the patient is asymptomatic conservative treatment is a good election and regular follow-up may be advised.
Venous aneurysm in the lower extremities
The clinical presentation of super?cial VAs in lower extremities is that of a palpable soft lump. It can change its size and location with the body’s position or the Valsalva maneuver; they can be completely asymptomatic or painful with edema.
The diagnosis can be made by the clinical history and physical examination in most cases, but it is usually con?rmed by image studies. Within image studies, vascular ultrasound is the method of choice for the study of Vas [62]. In the ultrasound, VAs are presented as an anechoic cystic structure, with well-de?ned walls, which can be sacular or fusiform and with a low ?ow volume; also, they provide us with information on vascular connections, the existence of thrombosis, or if there is an associated arteriovenous ?stula or any other pathologies [63], in addition to guiding the therapeutic approach. The CAT scan, the MRI and the phlebography are studies which can be performed in case of diagnostic doubt or when more exact information is required (size, extension, associated lesions and vascular origin) [64]. Clinical history and examination are the basis for reaching any diagnosis, but in the case of venous problems, vascular ultrasound evaluation is fundamental to reaching a correct diagnosis, and thus choosing the proper treatment. It is only in case of doubt, where further imaging studies are required. In the differential diagnosis, we must consider varicose veins, soft tissue tumors, lymphatic malformations, hemangiomas and, depending on location, inguinal hernias. VA complications are: thrombophlebitis, deep venous thrombosis, pulmonary embolism and bleeding caused by rupture.
Coagulation disorders associated with VAs are characterized by blood stasis in the dilated vessels and with a low blood ?ow, which activates the coagulation cascade with the subsequent production of thrombin and the conversion of ?brinogen into ?brin [65,66]. Then the ?brinolysis process begins which is re?ected in the rise in ?brinogen derived products, including D-dimer. This is the simpli?ed description of located intravascular coagulopathy which characterizes coagulopathy associated with venous malformations [67,68]. Newly formed microthrombus attach to calcium and phleboliths are formed [69,70]. These can be detected during physical examination and veri?ed by imaging studies. The presence of phleboliths in VAs can indicate treatment with anticoagulants, especially in large and extensive Vas [71]. Localized intravascular coagulopathy is of utmost importance because it is linked to the presence of local pain, thrombophlebitis, deep venous thrombosis and pulmonary embolism.
Even though deep venous aneurysms are more susceptible to presenting pulmonary embolism and their frequency is more common in this type of VA, super?cial VAs are not free from presenting this complication. Due to this, they must be treated with anticoagulant therapy, just as the deep ones. Regarding the risk of VA rupture, it represents something theoretical since there are no reported cases of this complication.
Super?cial VA treatment can be conservative, endovascular or surgical. If the VA is not too large and does not cause symptoms it may be treated conservatively through compressive therapy and prophylaxis in order to avoid thrombophlebitis or deep venous thrombosis. The indications for surgical treatment in super?cial VAs are: the presence of symptoms, the risk of thrombosis, compression of nearby structures and, more commonly, esthetic problems [72]. Surgical treatment consists of ligation and total excision of the aneurysm [73]. Ekim et al., [74] suggest surgery in all cases to prevent possible complications.
However, in some cases, endovascular methods can be used, like foam, laser or radiofrequency sclerotherapy [75]. In the case of patients who present super?cial venous thrombosis above the knee or deep venous thrombosis at any location, it is important to treat with anticoagulants for 3-6 months in patients with normal thrombophilic pro?les, otherwise the anticoagulant is prescribed inde?nitely. In the presented case, there is no doubt that the chosen surgical treatment is adequate and the treatment with anticoagulants due to the presence of deep thrombosis for a period of three months is acceptable.
The results of the treatment of these types of pathologies are excellent as long as they are not associated with other vascular malformations or pathologies; there are no reports of mortality in super?cial VA surgical intervention in the lower extremities and the morbidity is the same as any venous surgical procedure [76].
All our cases, were treated surgically, because in the majority of cases were symptomatic, and the rest of patients preferred surgical treatment because cosmetic reasons or fear to some complication appeared. We preferred surgical approach instead of endovascular procedures because the characteristic and location of the VA. The diagnosis was supported by DUS and no other studies were required. There was no complication and the outcome was excellent.
CONCLUSION
REFERENCES
- Lee BB, Laredo J, Lee TS, Huh S, Neville R (2007) Terminology and classification of congenital vascular malformations. Phlebology 22: 249-252.
- Belov ST (1990) Classification of congenital vascular defects. Int Angiol 9: 141-146.
- Lee BB, Baumgartner I, Berlien P, Bianchini G, Burrows P, et al. (2015) Diagnosis and Treatment of Venous Malformations. Consensus Document of the International Union of Phlebology (IUP): updated 2013. Int Angiol 34: 97-149.
- Lee BB, Antignani PL, Baraldini V, Baumgartner I, Berlien P, et al. (2015) ISVI-IUA consensus document diagnostic guidelines on vascular anomalies: vascular malformations and hemangiomas. Int Angiol 34: 333-374.
- Caggiati A, Bergan JJ, Glovickzi P, Eklof B, Allegra C, et al. (2005) Nomenclature of the veins of the lower limb: extensions, refinements, and clinical applications. J Vasc Surg 41: 719-724.
- Lee BB (2015) New classification of congenital vascular malformations (CVMs). Reviews in Vascular Medicine 3: 1-5.
- Lee BB (2015) All congenital vascular malformations should belong to one of two types: “truncular” or “extratruncular”, as different as apples and oranges! Phlebological Review 1: 1-3.
- Zamboni P, Cossu A, Carpanese L, Simonetti G, Massarelli G, et al. (1990) The So-Called Venous Aneurysms. Phlebology 5: 45-50.
- CL (1994) Curchill´s Medical Illustrated Dictionary. Churchill Livingstone, London, United Kingdom.
- Rodriguez HE, Pearce WH (2009) The management of venous aneurysms. (eds.) Glovicki P, Dalsing MC, Eklof B, Moneta GL, Wakefield TW, Handbook of venous disorders. London: Hodder Arnold Pg no: 604-616.
- Hilscher WM (1995) Zur Frage der venösen Aneurysmen. Fortscher Röntgenstr 82: 244-247.
- Mateo AM, Mateo Martínez M (1997) Aneurismas venosos. (eds.) Estevan Solano JM, Tratado de aneurismas. Barcelona: Uriach Pg no: 589-597.
- Van Der Stricht J (1988) Classification of vascular malformations. Phlebology: The Journal of Venous Disease 3: 203-206.
- Belov ST (1989) Classification, terminology, and nosology of congenital vascular defects. (eds.) Belov ST, Loose DA, Weber J, Vascular Malformations. Reinbek, Germany: Einhorn-Presse 25: 30.
- Belov St (1993) Anatomopathological classification of congenital vascular defects. Semin Vasc Surg 6: 219-224.
- Mattassi R, Loose DA, Vaghi M (2009) Hemangiomas and Vascular Malformations: An Atlas of Diagnosis and Treatment. Springer Verlag Mailand Pg no: 336.
- issva.org/classification.
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, et al. (2015) Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 136: 203-214.
- Harris RI (1928) Congenital venous cyst of the mediastinum. Ann Surg 88: 953-956.
- Schild H, Berg ST, Weber W. (1992) Das Veneanevrysma. Akt Radiol 2: 75-80.
- Gillespie DL, Villavicencio JL, Gallagher C, Chang A, Hamelink JK, et al. (1997) Presentation and management of venous aneurysmsJ Vasc Surg 26: 845-852.
- Rodriguez HE, Pearce WH (2009) The managment of venous aneurysm. (eds.) Glovicki P, Dalsing MC, Eklof BG, Handbook of venous disorders. London: Hodder Arnold Pg no: 604-616.
- McDevitt DT, Lohr JM, Martin KD, Welling RE, Sampson MG (1993) Bilateral popliteal vein aneurysms. Ann Vasc Surg 7: 282-286.
- Hamper UM, DeJong MR, Scoutt LM (2007) Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am 45: 525-548.
- Sessa C, Perrin M, Nicolini P (2005) Anévrismes veineux. EMC Chirurgie 2: 317-331.
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, et al. (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149-161.
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, et al. (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8: 211-216.
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, et al. (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87: 1161-1169.
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC , et al. (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171-1180.
- Kim I, Kim HG, So JN, Kim JH, Kwak HJ, et al. (2000) Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'- Kinase/Akt signal transduction pathway. Circ Res 86: 24-29.
- Irwin C, Synn A, Kraiss L, Zhang Q, Griffen MM, et al. (2008) Metalloproteinase expression in venous aneurysms. J Vasc Surg 48: 1278-1285.
- SCHATZ IJ, FINE G (1996) Venous aneurysms. N Engl J Med 266: 1310-1312.
- Lev M, Saphir O (1951) Endophlebohypertrophy and Endophlebosclerosis II. The external and common iliac veins. Arch J Pathol 51: 401-411.
- Lev M, Saphir O (1951) Endophlebohypertrophy and Endophlebosclerosis. I. The Popliteal Arch J Pathol 51: 154-178.
- Gruber W (1875) Phlebektasie unter der form eines varix von enormer grosse in vereingungswinkel der vena jugularis interna and und subclavia. Arch Path Anat 65: 227-229.
- de Oliveira Góes Junior AM, Monteiro Franco RM, de Campos Vieira Abib S (2014) Internal Jugular Vein Aneurysm Presenting after Emesis Episode. Angiol 2: 130.
- Andreev A, Petkov D, Kavrakov T, Penkov P (1998) Jugular venous aneurysms: When and how to operate. Int Angiol 17: 272-275.
- Sommer L, Forte V (2001) Congenital venous aneurysms of the internal jugular vein in a child. J Otolaryngol 30: 126-128.
- Indudharan R, Quah BS, Shuaib IL (1999) Internal jugular phlebectasia-an unusual case of neck swelling. Ann Trop Paediatr 19: 105-108.
- Kirmani S, Rashid M, Ali I, Badar F (2011) External jugular vein aneurysm: a rare cause of neck swelling. J Ultrasound Med 30: 1157-1158.
- Al-Shaikhi A, Kay S, Laberge JM (2003) Laberge External jugular venous aneurysm: an unusual cause of a neck mass in a young child. J Pediatr Surg 38: 1557-1559.
- Lee HY, Yoo SM, Song IS, Yu H, Lee JB (2007) “Sonographic diagnosis of a saccular aneurysm of the internal jugular vein. J Clin Ultrasound 35: 94-96.
- Sander S, Eliçevik M, Unal M, Vural O (1999) Vural Jugular phlebectasia in children: is it rare or ignored?. J Pediatr Surg 34: 1829-1832.
- Rawat NS, Gupta A, Khurana P, Jain S, Trehan N (2008) MSCT angiography diagnosis of thrombosis in external jugular venous aneurysm: case report and review of literature. Indian Heart J 60: 52-54.
- Porcellini M, Selvetella L, Bernardo B, Del Guercio L, Baldassarre M (1996) [Aneurysms of the external jugular vein]. G Chir 41: 238-241.
- Beale TJ, Smedly FH, Knee G (1996) Thrombosis within an external jugular venous aneurysm. J R Coll Surg Edinb 41: 181-182.
- Vematsu N, Okada M (1999) Primary Venous Aneurysms: Case Reports. Angiology 50: 239-244.
- Lubianca-Neto JF, Mauri M, Prati C (1999) Internal Jugular Phlebectasia in Children. Am J Otolarynngol 20: 415-418.
- Jianhong L, Xuewu J, Tingze H (2006) Surgical treatment of jugular vein phlebectasia in children. Am J Surg 192: 286-290.
- Jianhong L, Xuewu J, Tingze H (2005) Congenital jugular vein phlebectasia. Am J Otolaryngol 26: 172-174.
- Faraj W, Selmo F, Hindi M, Haddad F, Khalil I (2007) Cephalic vein aneurysm. Ann Vasc Surg 21: 804-806.
- Tahata T, Kusuhara K, Johno H, Okamoto K (2001) Venous Aneurysms of the upper extremity. A case report. Angiology 53: 479-481.
- Schellhammer F, Wobker G, Turowski B (2005) Asymptomatic aneurysm of the subclavian vein. Acta Radiol 46: 366-367.
- Wallace JR, Baril DT, Chaer RA (2013) Upper extremity venous aneurysm as a source of pulmonary emboli. Ann Vasc Surg 27: 240.
- Pedersen G, Laxdal E, Amundsen SR, Dregelid E, Jonung T, et al. (2005) Aneurysm of the subclavian vein. EJVES Extra 10: 89-91.
- McCready RA, Bryant MA, Divelbiss JL, Chess BA (2007) Subclavian venous aneurysm: case report and review of the literature. J Vasc Surg 45: 1080-1082.
- Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, et al. (2001) Soft-tissue venous malformations in adult patients: Imaging and therapeutic issues. Radiographics 21: 1519-1531.
- Paltiel HJ, Burrows PE, Kozakewich HP, Zurakowski D, Mulliken JB (2000) Soft-tissue vascular anomalies: Utility of US for diagnosis. Radiology 214: 747-754.
- Gabrielli R, Rosati MS, Siani A, Irace L (2012) Management of symptomatic venous aneurysm. Sci World J 2012: 386478.
- Seo SH, Kim MB, Kwon KS, Kim CW, Oh CK (2008) Primary venous aneurysms of the superficial venous system. Angiology 59: 593-598.
- Pascarella L, Al-Tuwaijri, Bergan JJ, Mekenas LM (2005) Lower extremity super?cial venous aneurysms. Ann Vasc Surg 19: 69-73.
- Labropoulos N, Volteas SK, Giannoukas AD, Touloupakis E, Delis K, et al. (1996) Asymptomatic popliteal vein aneurysms. Vasc Surg 30: 453-457.
- Wolosker N, Zerati AE, Nishinari K, de Melo Galvão Filho M, Wolosker AM (2004) Aneurysm of superior mesenteric vein: case report with 5-year follow-up and review of the literature. J Vasc Surg 39: 459-461.
- Watanabe A, Kusajima K, Aisaka N, Sugawara H, Tsunematsu K (1998) Idiopathic saccular azygos vein aneurysm. Ann Thorac Surg 65: 1459-1461.
- Hermans C, Dessomme B, Lambert C, Deneys V (2006) Venous malformations and coagulopathy. Ann Chir Plast Esthet 51: 388-393.
- Redondo P, Aguado L, Marquina M, Paramo JA, Sierra A, et al. (2010) Angiogenic and prothrombotic markers in extensive slow-?ow vascular malformations: implications for antiangiogenic/antithrombotic strategies. Br J Dermatol 162: 350-356.
- Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, et al. (2008) Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 144: 873-877.
- Mazoyer E, Enjolras O, Bisdorff A, Perdu J, Wassef M, et al. (2008) Coagulation disorders in patients with venous malformation of limbs and trunk: a study in 118 patients. Arch Dermatol 144: 861-867.
- Hein KD, Mulliken JB, Kozakewich HP, Upton J, Burrows PE, et al. (2002) Venous malformations of skeletal muscle. Plast Reconstr Surg 110: 1625-1635.
- Enjolras O, Wassef M, Mazoyer E, Frieden IJ, Rieu PN, et al. (1997) Infants with Kasabach-Merritt syndrome do not have "true" hemangiomas. J Pediatr 130: 631-640.
- Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L (2002) Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol 24: 243-251.
- Sessa C, Nicolini P, Perrin M, Farah I, Magne JL, et al. (2000) Management of symptomatic and asymptomatic popliteal venous aneurysms: a retrospective analysis of 25 patients and review of the literature. J Vasc Surg 32: 902-912.
- Friedman SG, Krishnasastry KV, Doscher W, Deckoff SL (1990) Primary venous aneurysms. Surgery 108: 92-95.
- Ekim H, Kutay V, Tuncer M, Gultekin U (2004) Management of primary venous aneurysms. Saudi Med J 25: 303-307.
- Lee BB (2013) Endovascular management of the Congenital Vascular Malformation (CVM) is not a panacea. Editorial. Damar Cer Derg 22: 1-3.
- Roh YN, Do YS, Park KB, Park HS, Kim YW, et al. (2012) The results of surgical treatment for patients with venous malformations. Ann Vasc Surg 26: 665-673.
Citation: Elías FGR, Rasgado FV, Sánchez MH, Danés LG (2019) Truncular Venous Malformations: Venous Aneurysm Case Series in a General Hospital. J Angiol Vasc Surg 4: 023.
Copyright: © 2019 Felipe Gerardo Rendón Elías, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

