
Tuberculous Meningitis - An Entity Not to Forget
*Corresponding Author(s):
Lídia LeiteDepartment Of Pediatrics, Hospital De Braga, Sete Fontes, Braga, Portugal
Tel:+351 918087169,
Email:lidialeitemarques@gmail.com
Abstract
A 27-month-old child was admitted with fever, anorexia, convergent strabismus and irritability. Cerebrospinal fluid was suggestive of tuberculous meningitis. Anti-tuberculosis treatment was initiated and a ventricular shunt for hydrocephalus was placed, with clinical improvement. We aim to alert to this rare and severe condition, with a high mortality rate.
Keywords
Convergent strabismus; Meningitis; Pediatrics; Sixth nerve palsy; Tuberculous
ABBREVIATIONS
BCG: Bacillus Calmette-Guérin
CNS: Central Nervous System
CSF: Cerebrospinal Fluid
CT: Computed Tomography
DNA: Deoxyribonucleic Acid
ED: Emergency Department
EVD: External Ventricular Shunt
GCS: Glasgow Coma Score
HIV: Human Immunodeficiency Virus
Mt: Mycobacterium Tuberculosis
PCR: Polymerase Chain Reaction
PICU: Pediatric Intensive Care Unit
TB: Tuberculosis
WHO: World Health Organization
INTRODUCTION
Tuberculosis (TB) is a major public health problem. One third of the world’s population is infected with Mycobacterium Tuberculosis (Mt), which progresses to active disease in approximately 10% of individuals [1,2]. In 2015, the World Health Organization (WHO) estimated that 10.4 million cases of TB occurred worldwide, of which 10% in children [1]. Tuberculous meningitis corresponds to 1-2% of cases of active tuberculosis [1,3,4]. In Portugal, the annual incidence of tuberculous meningitis in children aged less than 5 years was less than 1:10.000.000 inhabitants between 2012 and 2016, one of the WHO criteria for control of TB [5]. It is the most serious manifestation of Mt infection and represents a medical emergency [1-4,6-10]. Approximately one half of tuberculous meningitis patients die or suffer severe neurologic disability, with 100% mortality rate if left untreated [1,2]. The clinical staging system was developed by the British Medical Research Council: Stage 1 - conscious, oriented without focal neurological deficits; stage 2 - focal neurological deficit or Glasgow Coma Score (GCS) of 10 - 14; stage 3 - GCS <10. The clinical stage on presentation is a strong prognostic factor, with a high mortality risk for patients presenting with stage 3 disease [1,2,3,8].
The diagnosis can be difficult because of non-specific clinical features and lack of a readily available sensitive test. Cerebrospinal Fluid (CSF) analysis is crucial to the diagnosis. Prompt initiation of anti-tuberculosis treatment for all suspected cases remains a key aspect of management, because treatment delay is associated with worst prognosis.
We present the case of a child with meningitis due to Mt. To our knowledge, there are no similar studies published in the Portuguese population.
CASE REPORT
A previously healthy 27-month-old caucasian female child, was admitted to the Pediatric Emergency Department (ED) with an 8-day history of low-grade fever, food refusal, sore throat and bilateral convergent strabismus. She had lost weight in the last two months, dropping from the 15-50th percentile to the 3-15th percentile. The national vaccination program was updated, without Bacillus Calmette-Guérin (BCG) vaccine at birth [5]. There was no family history of TB.
Physical examination revealed a pale-looking, irritable child, with stable vital signs. On neurological examination she had a GCS 14 (O4V4M6), negative meningeal signs and bilateral convergent strabismus, most evident on the left.
A complete blood count showed hemoglobin 138g/L, leukocyte 14,6x109/L - neutrophils 8,6x109/L, lymphocytes 4,7x109/L and platelet 324x109/L. The C-reactive protein level was 3,48mg/L. Serum electrolytes revealed a hyponatremia of 130mmol/L. Chest radiograph did not show any abnormality. Brain Computed Tomography (CT) and magnetic resonance imaging showed discrete subarachnoid exudate, with left temporo-parieto-occipital and intraventricular predominance, decanted in the occipital horns of the lateral ventricles with minimal associated enhancement; discrete dilation of the ventricular system, such as deletion of the convexity grooves (Figure 1).
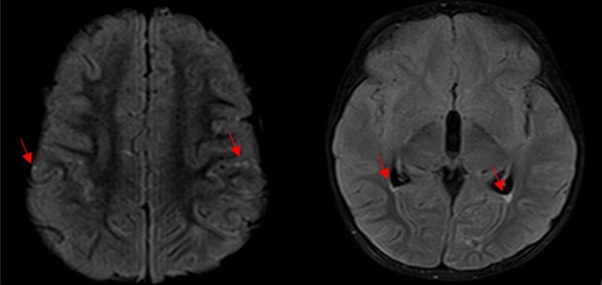 Figure 1: With left temporo-parieto-occipital and intraventricular predominance, decanted in the occipital horns of the lateral ventricles with minimal associated enhancement; discrete dilation of the ventricular system, such as deletion of the convexity grooves.
Figure 1: With left temporo-parieto-occipital and intraventricular predominance, decanted in the occipital horns of the lateral ventricles with minimal associated enhancement; discrete dilation of the ventricular system, such as deletion of the convexity grooves.
Lumbar puncture revealed an elevated opening pressure (36 cmH2O) and the CSF examination showed lymphocytic pleocytosis (155 cells with 76% lymphocytes), increased protein (1,08mg/dL) and decreased glucose (8mg/dL) concentrations. Adenosine deaminase level in CSF was of 5,9U/L (normal). Ceftriaxone, ciprofloxacin and acyclovir, were administered empirically for bacterial meningitis/viral encephalitis and the child was admitted in the Pediatric Intermediate Care Unit. Ophthalmology observation confirmed the bilateral sixth nerve palsies. Despite therapy, fever persisted and vancomycin was added to treatment. The child had progressive neurologic impairment in the first 48h of admission, with fluctuating levels of consciousness (GCS 10-11) and hypertension without bradycardia. Given the CSF findings, the diagnosis of tuberculous meningitis was considered and therapy with isoniazid, rifampicin, pyrazinamide, etambutoland intravenous dexamethasone is started. Brain CT showed greater dilation of the ventricular system, reflecting hydrocephalus in evolution, reason why she was transferred to a Pediatric Intensive Care Unit (PICU) and a long-term External Ventricular Shunt (EVS) was placed. She had good clinical response and was discharged from the PICU after 8 days. Ciprofloxacin and vancomycin were suspended and she completed 7 days of treatment with acyclovir and 10 days of ceftriaxone.
Interferon-gamma release assay was positive. Viral serologies, including HIV, bacterial cultures of the blood, urine and CSF were all negative. The CSF culture was positive for Mt - sensitive to all four anti-tuberculosis. CSF Polymerase Chain Reaction (PCR) could not detect Mt DNA. Gastric aspirate culture and PCR were also negative for Mt.
As the clinical and neurologic condition improved, the EVS was removed after 10 days and she completed 2 weeks of intravenous dexamethasone switching to oral prednisolone. After 30 days of anti-tuberculosis treatment, she was discharged home, only maintaining bilateral convergent strabismus, which improved progressively over 12 months. At the time of discharge, she kept corticoid therapy in a weaning scheme and anti-tuberculosis treatment, having completed 2 months of quadruple therapy, maintaining isoniazid and rifampicin until completing 12 months of treatment. The response to treatment was excellent with no residual neurological deficits and appropriate morphology and dimensions of the ventricular system on follow-up brain CT.
DISCUSSION
After inhalation of respiratory droplets with bacilli into distal airways, the Mt spreads to the local draining lymph nodes in the lung and then to distant sites during hematogenous dissemination [1,7]. The Mt can be deposited adjacent to the cerebral ventricles or subarachnoid space, leading to granuloma formation [1]. Postmortem studies [11], suggest that the rupture of granulomas, adjacent to the subarachnoid space, causes a strong inflammatory response - the triggering event of tuberculous meningitis [1,7]. The infection of the Central Nervous System (CNS) can manifest itself as acute, subacute or chronic meningitis or by space-occupying lesions - tuberculomas [1]. CNS infection can occur on its own or associated with pulmonary or disseminated disease [1].
The prevalence of tuberculous meningitis is higher between 2-4 years old. Hydrocephalus is the most common complication. Two thirds of the cases have neuro-ophthalmological disorders (retrobulbar optic neuritis and optic atrophy) - Mt can invade the optic nerve or accumulate tuberculous exudates [1,7].
The duration of symptoms may range from 1 day to 6 months [1]. The prodromal symptoms are nonspecific, and include malaise, anorexia, vomiting, headache and fever. Acute presentations may be difficult to distinguish from other forms of bacterial meningitis [1]. Sixth nerve palsies are the most common at the time of initial presentation but other cranial nerve may be involved. In our case, fever, anorexia and malaise were predominant, followed by sixth nerve palsy. Hydrocephalus, seizures, pyramidal or cerebellar signs, progressive dementia with personality change, coma and death can occur throughout the diagnostic and treatment period [1,3,4,6,8,12].
On lumbar puncture, the opening pressure is usually moderately elevated and typical CSF findings include lymphocytic pleocytosis, increased protein and decreased glucose concentrations, similar to our results. The sensitivity of CSF culture is very low. PCR to detect Mt has high specificity but low sensitivity.
Despite the fact that neuroimaging study may be normal in the initial stage of the disease, the typical findings include hydrocephalus, cerebral infarction, localized or disseminated stenosis of intracranial arteries and tuberculomas [1,2,3,8-10,12].
Hyponatremia is caused by the syndrome of inappropriate antidiuretic hormone secretion or by the cerebral salt-wasting syndrome. In this case, the cause appeared to be cerebral salt-wasting syndrome, once she presented hyponatremia associated with increased urinary osmolarity with high excretion of urinary sodium and polyuria. Anti-tuberculosis treatment is the same as that used for adults. Guidelines from the Infectious Disease Society of America and US Centers for Disease Control and Prevention recommend initial four-drug therapy with isoniazid, rifampin, pyrazinamide, and ethambutol. Pyrazinamide is discontinued after 2 months of therapy and ethambutol after confirmation of Mt sensitivity to isoniazid. The total duration of treatment suggested is 9 to 12 months. Adjuvant glucocorticoid therapy is recommended for all patients [1,3]. Regarding the treatment of complications, in the specific case of hydrocephalus, a long-term EVS should be placed [2]. The immediate onset of treatment in all suspected cases is essential, given the strong relationship between the stage of the disease at the beginning of treatment and the long-term prognosis [1,3,4,6,8,12].
Since 1965, the BCG vaccinewaspartofthe Portuguese NationalVaccinationProgram. As Portugal assisted a progressive reduction in TB incidence and reached the threshold to be considered a low incidence country (20/100,000 inhabitants) [5] universal vaccination was suspended on 2017, with only few exceptions [5]. The BCG vaccine provides protection against severe forms of the disease in children, namely meningitis, but it doesn´t protect against primary infection or reactivation of the disease.
In 2018, the incidence of TB in Portugal continued to decrease and was 15,4/100,000 inhabitants with 34 cases reported in children aged 5 years or less, corresponding to an incidence of 6,59 cases/100,000 children in this age group [13]. Four cases of severe forms of TB were identified, all in children without BCG vaccine, with three of them with individual criteria for vaccination [13].
With this case report, we aim to alert to this rare condition that has a high mortality and complication rate, and to emphasize the importance of early diagnosis, treatment, and BCG vaccine in the prevention of severe forms of the disease. To keep the strategy of selective vaccination, it is important to identify children belonging to risk groups and their referral for vaccination as soon as possible and to adequate track cases to avoid transmission and the development of the disease.
ARTICLE SUMMARY
Tuberculous meningitis corresponds to 1-2% of cases of tuberculosis. Approximately one half patients die or suffer severe neurologic disability, with 100% mortality if left untreated.
CONTRIBUTORS’ STATEMENT PAGE
Dr. Lídia Leite, Dr. Ivo Miguel Neves and Dr. Filipa Balona were in the emergency department at the time of hospital admission, and revised the manuscript.
Dr. Lídia Leite, Dr. Liliana Branco and Dr. Ângela Oliveira and Dr Marina Pinheiro followed the hospitalization in the pediatric intermediate care unit, and revised the manuscript.
Dr. Marta Grilo accompanied the hospitalization in the pediatric intensive care unit during the clinical worsening, and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENT
All participants in the preparation of the article.
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflicts of interest relevant to this article to disclose.
FUNDING/SUPPORT
No funding was secured for this study.
ROLE OF FUNDER/SPONSOR
The funder/sponsor did not participate in the work.
REFERENCES
- Mezochow A, Thakur K, Vinnard C (2017) Tuberculous meningitis in children and adults: New insights for an ancient foe. Curr Neurol Neurosci Rep 17: 85.
- Anjum N, Noureen N, Iqbal I (2018) Clinical presentations and outcomes of the children with tuberculous meningitis: An experience at a tertiary care hospital. J Pak Med Assoc 68: 10-15.
- Leonard JM (2017) Central nervous system tuberculosis. Microbiol Spectr 5.
- Miftode EG, Dorneanu OS, Leca DA, Juganariu G, Teodor A, et al. (2015) Tuberculous meningitis in children and adults: A 10-year retrospective comparative analysis. Plos One 10: 0133477.
- DGS (2016) Estratégia de vacinação contra a tuberculose com a vacina BCG. DGS, Lisboa, Portugal. Pg no: 1-9.
- van Toorn R, Solomons R (2014) Update on the diagnosis and management of Tuberculous meningitis in children. Seminars in Pediatric Neurology 21: 12-18.
- Be NA, Kim KS, Bishai WR, Jain SK (2015) Pathogenesis of central nervous system tuberculosis. CurrMol Med PMC 9: 94-99.
- Cherian A, Thomas SV (2011) Central nervous system tuberculosis. Afr Health Sci 11: 116-127.
- Christie LJ, Loeffler AM, Honarmand S, Flodd JM, Baxter R, et al. (2008) Diagnostic challenges of central nervous system tuberculosis. Emerging Infectious Diseases 14: 1473-1475.
- Takahashi T, Tamura M, Takasu T (2012) The PCR-based diagnosis of central nervous system tuberculosis: Up to date. Tuberc Res Treat 2012: 831292.
- Rich AR, McCordock HA (1933) Pathogenesis of tubercular meningitis. Bull Johns Hopkins Hosp 52: 5-13.
- Kuo VC, Sloan LM, Emmett M (2010) Central nervous system tuberculosis. Baylor University Medical Center Proceedings 23.
- DGS (2019) Tuberculose em Portugal 2018, (dados provisórios), Programa Nacional para Tuberculose. DGS, Portugal.
Citation: Leite L, Neves IM, Oliveira Â, Branco L, Balona F, et al. (2021) Tuberculous Meningitis - An Entity Not to Forget. J Neonatol Clin Pediatr 8: 065.
Copyright: © 2021 Lídia Leite, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

