
Twin D-Frail Study Protocol: Does Vitamin D Link Vascular Endothelial Dysfunction to Sarcopenic Frailty
*Corresponding Author(s):
Amarasekera ATBlacktown Clinical And Research School, Western Sydney University And Western Sydney Local Health District, Level 2, Marcel Crescent, Blacktown, New South Wales, 2148, Australia; Department Of Cardiology, Blacktown Hospital, Western Sydney Local Health District, Sydney, Australia, School Of Nursing And Midwifery, Western Sydney University, Blacktown Hospital, Sydney, Australia
Tel:+61 298516063,
Email:Anjalee.Amarasekera@health.nsw.gov.au
Abstract
Background: A low vitamin D state and vitamin D deficiency is common in the elderly and has been correlated with various cardiovascular risk factors and events. Vitamin D deficiency has been demonstrated to play a major role in the pathogenesis of physical frailty and hypothesized to contribute to physical frailty through sarcopenia (the loss of skeletal muscle mass and function with aging). Sarcopenia has been shown to be a major contributor to morbidity in older adults. One possible mechanism for this association could be the effect of vitamin D on subclinical vascular function (vascular endothelial dysfunction; VED). Recent data highlight the role of increased inflammation and oxidative stress coexist in VED and sarcopenic modulated frailty which implicates chronic inflammation as a major component in the pathogenesis of frailty. Vitamin D supplementation has been shown to down-regulate mediators of Vascular Endothelial Dysfunction (VED) and correct the associated inflammatory status hence may be a simple strategy for the treatment and prevention of frailty.
Study objectives: The proposed research study is designed to investigate anti-inflammatory effects of vitamin D on physical frailty, sarcopenia and VED. The 2 main aims of the study are: 1) To establish a relationship between VED and sarcopenia-related physical frailty in a cohort of patient with cardiovascular conditions and 2) To investigate the effects of vitamin D supplementation in improving vascular endothelial function by correcting inflammatory and oxidative stress profiles and thus improve muscle tone in healthy working adults.
Method/design: The proposed study protocol will comprise of two inter-related study arms. The first arm of the study is aimed at addressing the first aim using a single-centred, prospective cross-sectional analytical design. This arm will also investigate the independent effects of vitamin D in the relationship of physical frailty and sarcopenia with VED. The second arm of the study will address the second aim. The second arm will adopt a pilot approach focusing on delineating the effects of down-regulation of inflammation-mediated pathways affecting muscle strength and function. In particular, the impact of vitamin D supplementation on Thrombospondin-1 (TSP-1; mediator of VED) and Tumor Growth Factor β1 (TGF-β1: modulator of inflammation) will be measured in healthy working adults.
Discussion: The hypothesis that vitamin D deficiency causes frailty via its inflammation related VED, which in turn leads to sarcopenia and physical frailty, will be explored. Novel frailty management strategies are essential to improve the quality of life of frail people and reduce the healthcare cost associated with frailty outcomes. The proposed research study will introduce a frailty management strategy via prevention/improvement of inflammatory status and early-stage subclinical CVD (VED) by simple and safe treatment strategy, vitamin D supplementation in older frail adults. The outcomes of the clinical intervention could be translated to better health outcomes by implementing frailty prevention/controlling strategy at early stages, before other CV complications occur by reducing the negative impact on patient’s quality of life and the healthcare systems, which will assist to reduce socioeconomic burden and improve quality of life of older people. This study will further investigate underlining mechanisms of association between vascular endothelial dysfunction and sarcopenia-related physical frailty. Positive outcome of the mechanistic study would provide baseline biomedical mechanistic insight for further studies to introduce new pharmacological interventions in frailty.
Clinical trial registration: This trial is registered in Australia New Zealand Clinical Trial Registry ACTRN12621000059864. The WHO Universal Trial Number (UTN): U1111-1254-3879.
Keywords
Inflammation; Physical frailty; Sarcopenia; Vascular endothelial dysfunction; Vitamin D
Background
Low vitamin D status and vitamin D deficiency is common in the elderly and has been correlated with various cardiovascular risk factors and events. Large observational studies demonstrate that low and high levels of 25(OH)D were associated with cardiovascular disease, stroke, and acute myocardial mortality in a nonlinear, reverse J-shaped manner with the highest risk at lower levels of vitamin D [1,2]. Vitamin D deficiency has been demonstrated to play a major role in the pathogenesis of physical frailty (aging-associated decline in strength and physiological reserve) and it has been hypothesized to contribute to physical frailty through sarcopenia (the loss of skeletal muscle mass and function with aging). Frailty is a major public health problem affecting the aging population. It is associated with poor health-outcomes including disability, hospitalization, and mortality with significantly higher healthcare utilization and related economic cost. Novel frailty management strategies are essential to improve the quality of life of frail people and reduce the healthcare cost associated with frailty outcomes.
Sarcopenia-related physical frailty and subclinical cardiovascular disease
Frailty is strongly associated with adverse cardiovascular outcomes; however, the mechanisms underlying the pathophysiology of this association remain largely unknown. Recent data has demonstrated a positive association between Vascular Endothelial Dysfunction (VED: the earliest form of subclinical cardiovascular diseases) and physical frailty [3,4]. These studies suggest that VED may significantly contribute to the development of sarcopenia (characterised by loss of skeletal muscle mass and function with advancing age), a major contributor to physical frailty and morbidity in older adults [5].
Cross-linking common underlying mechanisms of inflammation and oxidative stress in VED and physical frailty
Aging has been associated with an inflammatory state and oxidative stress. The advancing age-associated elevated inflammatory state has been reported to coexist in both sarcopenia related frailty status and VED. Nitric Oxide (NO) signalling pathway is mainly responsible for vasodilation and vascular function [6]. Decreased NO (endothelial-derived vasodilator) availability, increased inflammation and oxidative stress are reported as key elements in the impairment of vascular endothelial function associated with advancing age. A heightened inflammatory state and oxidative stress have also been related to elevated levels of inhibitors of NO signalling pathway via Endothelial Nitric Oxide Synthase (eNOS) (the enzyme critical for the generation and bioavailability of NO and endothelial function). Increased inflammatory states and oxidative stress inhibit enzymes responsible for clearance of inhibitory plasma substances of eNOS activity, such as Asymmetric Dimethylarginine (ADMA), a methylated arginine residue, which are a strong mediator and marker of VED. Dimethylarginine Dimethylaminohydrolase (DDAH) is the enzyme involved in hydrolytic degradation of the ADMA to citrulline and methylamines. Literature showed that inhibition of DDAH caused an accumulation of intracellular ADMA and thereby indirectly inhibited the activities of eNOS [7]. Elevated levels of inflammation and oxidative stress can impair the normal activities of DDAH, which leads to an elevation of serum ADMA levels and inhibition of endothelium-derived synthesis of NO [8].
Association of low plasma vitamin D (25(OH)D3) with VED, sarcopenia-related physical frailty and inflammation
Low vitamin D status or vitamin D deficiency (defined generally as a 25(OH)D3 level < 20 ng/ml/50 nmol/l) is common in older persons. The 25(OH)D3 (circulating form of vitamin D) is considered the best biomarker of vitamin D status in the clinical setting. Increased oxidative stress and impaired vascular endothelial function have been suggested to be partly responsible for the adverse cardiovascular outcomes associated with low vitamin D in both animal and human studies [9-11]. A number of previous experimental findings raise the possibility that vitamin D may increase the activity and expression of eNOS (or nitric oxide synthase III), the enzyme critical to the generation and bioavailability of NO. For example, treatment of endothelial cells with calcitriol significantly reversed advanced glycation end product-induced down-regulation of endothelial NO synthase mRNA and activity [12]; aortic eNOS expression and urinary nitrate/nitrite excretion were reduced in vitamin D receptor knockout mice [11]. An animal study carried out in spontaneously hypertensive rats found that chronic treatment with active vitamin D metabolite reduced the endothelium-dependent vascular contractions in the aorta, resulting in reduction of blood pressure, basal reactive oxygen species and COX-1 expression [13]. Furthermore, Vitamin D Receptor (VDR) expression and 1α-hydroxylase (enzyme responsible for activating 25OHD3 to active form) mRNA and protein expressions have been described in endothelial cells [14]. Collectively, these results indicate positive role of vitamin D in regulating NO bioavailability in endothelial cells. Clinically, vitamin D levels positively correlated with vascular endothelial function. Randomised controlled studies demonstrated the positive effects of vitamin D supplementation on vascular function [9,15,16]. It was demonstrated in an ageing population (n=254), that low 25(OH)D3 levels are associated with increased plasma concentrations of ADMA (Ngo, D.T. et al., 2010). This relationship persisted even after adjustments for season, age, Body Mass Index (BMI) and other cardiovascular risk factors. Vitamin D may down regulate the inflammation and oxidative stress [17,18] by increasing the activity and mRNA expression of eNOS, the enzyme critical for the generation and bioavailability of NO and endothelial function, and down regulate the inflammation and oxidative stress. Despite the positive outcomes of vitamin D in VED and inflammation/oxidative stress, some studies demonstrated negative or nil effects of vitamin D on vascular function and inflammation/oxidative stress [19,20].
Previously, vitamin D supplementation has been introduced in clinical interventions to reduce sarcopenia related physical frailty [21]. However, the role of vitamin D in sarcopenia has also been controversial, as some observational studies have reported negative outcomes of vitamin D replacement on sarcopenia and physical function [22]. Moreover, vitamin D deficiency / insufficiency play a major role in secondary hyperparathyroidism, which may also indirectly affect VED and sarcopenia/muscle function via elevated levels of inflammation. Literature demonstrates a correlation between elevated Parathyroid Hormone (PTH) levels and VED in an aged obese-diabetic cohort with significant inflammatory status [23].
Underlining mechanisms of association between sarcopenia and VED - role of anti- inflammatory effects of vitamin D
Higher plasma concentrations of inflammatory markers (Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α)) are associated with lower muscle mass and lower muscle strength even in healthy older persons without significant cardiovascular diseases [24]. Elevated inflammatory cytokine levels, as often observed in healthy older persons, is proposed to contribute to the loss of muscle mass and strength that accompanies aging, even before other comorbidities are involved in the process. These results are consistent with the reported recent observations on the association of sarcopenia-related frailty and initial stage vascular dysfunction/VED, both conditions are characterised by elevated inflammation and oxidative stress.
Are ADMA and TSP-1 novel biomarkers of sarcopenia and physical frailty?
Asymmetric Dimethylarginine (ADMA) and Thrombospondin-1 (TSP-1) are potent inhibitors of NO signalling pathway via Endothelial Nitric Oxide Synthase (eNOS) (the enzyme critical for the generation and bioavailability of NO and endothelial function). Asymmetric Dimethylarginine (ADMA) is a methylated arginine residue, which has been characterized as an endogenous, competitive inhibitor of eNOS [25]. Vallance et al., [26] were the first to report that ADMA acts as endogenous inhibitors of eNOS. ADMA inhibits vascular NO production in pathophysiological concentrations by competitive interaction with L-arginine for eNOS enzyme. Dimethylarginine Dimethylaminohydrolase (DDAH) is the enzyme involved in hydrolytic degradation of the ADMA to citrulline and methylamines. DDAH activity in endothelial cells is decreased in elevated inflammatory status and high oxidative stress [7] which causing accumulation of ADMA. TSP-1 is large, trimetric, glycoprotein, which has been shown to be a negative regulator of NO signalling [27,28]. TSP-1 has been shown to be an inhibitor of Soluble Guanylate Cyclase (sGC), main component of NO signaling pathway thus blocking NO-cGMP signaling [29]. Small concentrations of TSP-1 have been reported to inhibit NO-stimulated activation of sGC in platelets [28]. ADMA and TSP-1 are mediators and strong plasma markers of many aspects of VED. High inflammatory status and oxidative stress associated with advancing age have been related to elevated levels of ADMA and TSP-1, which could contribute to further worsening of the related adverse conditions including frailty and CVD. Our previous study [30] demonstrated for the first time, a significant inverse correlation between plasma vitamin D levels and TSP-1 concentrations in relatively healthy subjects with mildly hypovitaminosis condition. In this study, vitamin D intervention for 12 weeks significantly increased the plasma vitamin D levels and down-regulated the TSP-1 concentrations by 2.5 folds together with both systolic and diastolic blood pressure levels. Therefore, it is entirely possible that the effects of vitamin D in suppressing TSP-1 could have dual beneficial effects: 1) improving vascular NO signalling and 2) prevention of TSP-1 activation of Tumor Growth Factor β1 (TGF-β1)-mediated pro-inflammatory signalling. In skeletal muscles, TGF-β1 plays several roles including stimulation of inflammatory response by inducing the synthesis of Tumor Necrosis Factor-α (TNF-α) in monocytes. Overproduction of TGF-β1 causes weaknesses in muscle function. Figure 1 explains the pathways for possible association between sarcopenia-related physical frailty and VED, in the presence of low vitamin D levels or vitamin D deficiency. 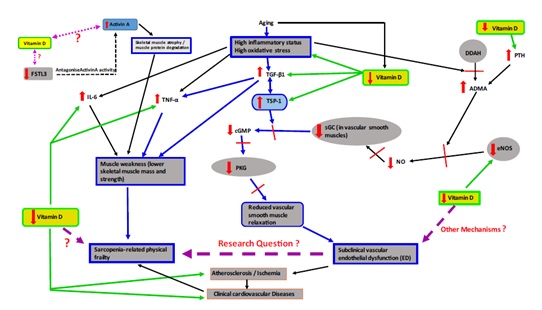
Figure 1: Possible association between sarcopenia-related physical frailty and ED.
ADMA: Asymmetric Dimethylarginine, cGMP: Cyclic Guanosine Monophosphate, eNOS: Endothelial Nitric Oxide Synthase, FSTL3: Follistatin-like 3, IL-6: Interleukin-6, PKG: Protein Kinase G, PTH: Parathyroid Hormone, sGC: Soluble Guanylyl Cyclase, TSP-1: Thrombospondin-1, TGF-β1: Tumor Growth Factor β1, TNF-α: Tumor Necrosis Factor-α) - It is possible that inflammation-induced TGF-β1 - TSP-1 axis (in blue colour in the diagram) could be one of the mechanisms links ED to sarcopenia-related physical frailty (hypothesis of the proposed research program)
Study Objectives
Primary objective - anti-inflammatory effects of vitamin D
The overall objective of our study is to examine the role of vitamin D in modulating the association between sarcopenia-related physical frailty and VED. As described in background section of this paper, vitamin D is reported to play major role in physical frailty and sarcopenia as well as in VED. Both these conditions are mediated by inflammation, hence our primary focus will be the anti-inflammatory effects of vitamin D in this association. We will primarily focus on the anti-inflammatory effects of vitamin D on the TGF-β1 - TSP-1 axis. To the best of our knowledge, this will be the first study to examine the role of plasma vitamin D in this emerging concept of VED and physical frailty and sarcopenia.
The research questions are 1) Does inflammation mediate the association between sarcopenia/physical frailty and VED in elderly or does VED act as an independent physiological correlate of sarcopenia/frailty; 2) What is the role of vitamin D in relation to prevention of frailty/sarcopenia.
Research hypothesis
In this proposed research, we hypothesise that; (1) the reported association between sarcopenia-related physical frailty and VED, could be partly mediated through the TSP-1 - TGF-β1 pathway; (2) vitamin D supplementation could prevent TGF-β1 induced increase in TSP-1 in subjects with vitamin D deficiency, and prevent inflammation-induced VED and sarcopenia-related physical frailty.
Secondary objective - association between physical frailty and cognitive frailty
As a secondary objective, this study will investigate association between physical frailty and cognitive frailty. Cognitive frailty is a new emerging concept in frailty research. Cognitive frailty is a condition recently defined by operationalised criteria describing coexisting physical frailty and Mild Cognitive Impairment (MCI). The cognitive frailty status is stratified into 2 subtypes; (a) potentially reversible cognitive frailty (physical frailty plus MCI); (b) reversibles cognitive frailty (physical frailty plus pre-MCI subjective cognitive decline). MCI is generally defined as significant memory loss without the loss of other cognitive functions. People with MCI are able to function independently and do not show other signs of dementia, such as impaired reasoning or judgment. Although patients with MCI are at greater risk of developing dementia compared with the general population, there is currently substantial variation in risk estimates (from <5% to 20% annual conversion rates), depending on the population studied. MCI is defined as a “symptomatic, pre-dementia phase” of the trajectory of cognitive decline [31].
A growing body of epidemiological evidence suggests that physical frailty may increase the risk of cognitive decline. Similarly, cognitive impairment may increase the risk of physical frailty, which suggests that physical frailty and cognitive frailty interact at advancing age [32]. The proposed research study will investigate the association of cognitive frailty with physical frailty and VED, as secondary end points.
Ethical considerations
The study is approved by the Human Research Ethics Committee (HREC) and governance of Western Sydney Local Health District (WSLHD), Sydney, Australia (2020/ETH01551) on 18th August 2020 and HREC of Western Sydney University, Sydney, Australia has recognised external ethics approval for the proposed project by WSLHD HREC;s (RH14015) on 21st August 2020. The study will be conducted in accordance with the declaration of Helsinki in its current applicable version and the guidelines of the International Conference on Harmonization of Good Clinical Practice (ICH-GCP) and the NHMRC (National Health and Medical Research Council, Australia), National Statement on ethical Conduct in Human Research (2007). Written informed consent will be taken from all participants before the study participation. All investigators and supporting staff will be ICH-GCP trained and certified. The trial will be monitored according to the ICH-GCP guidelines. This trial is registered in Australia New Zealand Clinical Trial Registry ACTRN12621000059864.
At the time of enrolment to the study, the participants will be given a unique study identification number, which will be used to identify the participants throughout the study, until study completion. No identifiable information will be used in the records. Study master list with identifiers will be securely kept separately from the list of unique study IDs given to the participants and study data records.
Participation in this study is entirely voluntary and the participants are not obliged to be involved. If the participant decides to withdraw their consent, they can withdraw at any time without the need to provide a reason. Should a participant become distressed during any time of the study, they would be provided with the option to exit study and they will be provided with the contact details of free confidential counselling services. All this information will be clearly outlined in the Participant Information Sheet (PIS) which will be provided to each participant.
Method/Design
The present study protocol consists of inter-related two sub-studies. The study 1 is a prospective cross-sectional analytical study to establish the newly identified association between physical frailty and sarcopenia with VED in patients with cardiovascular risk factors. The study 2 is single-armed, vitamin D intervention to investigate the independent effects of anti-inflammatory role of vitamin D on this association. Figure 2 represents the study flow. The sample size calculation and research methodology development have been carried out with the consultation of biostatistician and local health districts’ human research ethics/governance office. 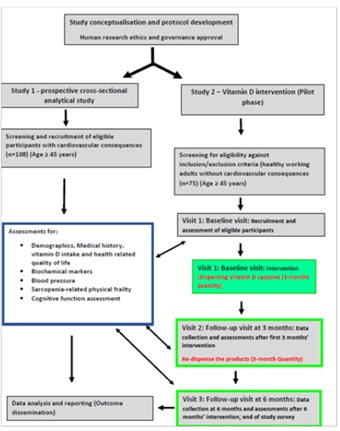
Figure 2: The d-frail study flow
Sample size calculation
Study 1: To detect the true difference of sarcopenia-related frailty in frail vs. non-frail individuals with 80% power and moderately large effect size 0.7, p=0.05, 2-tailed, total of 108 individuals with cardiovascular comorbidities (age>45 years) will be required to enrol at screening stage. This number will allow for a potential 20% drop-out rate.
Study 2: This pilot study will recruit 75 healthy working adults from university hospital.
Participants and settings
Study 1 participants’ inclusion and exclusion criteria and recruitment
Study 1 participants will be patients diagnosed with cardiovascular disease condition and referred to cardiology outpatient clinics of tertiary acute hospital. Participants will be invited to participate via poster/flyer displayed at the site and first consecutive patients, whose age ≥45 years, who are willing participate the study will be recruited. Consecutive recruitment will be carried out. Exclusion criteria include, diagnosed renal/liver diseases or musculoskeletal disorders, severe cognitive impairment (i.e., dementia/alzheimer’s disease) or pregnant or breastfeeding at the time of screening. This study will establish the relationship between VED and sarcopenia-related physical frailty in cardiovascular patient cohort and will investigate the independent effects of vitamin D in this relationship.
Study 2 participants’ inclusion and exclusion criteria and recruitment
For the second arm of the study, age-matched healthy participants will be recruited. Study 2 will investigate the effects of vitamin D intervention in improving vascular endothelial function by correcting inflammatory and oxidative stress profiles and improving muscle weakness in healthy working adults. This arm of the study will examine if down-regulation of TSP-1: TGF-β1 pathway by vitamin D supplementation could affect parameters of muscle strength and function. The participants will be healthy working adults, whose age ≥ 45 years and free from other cardiovascular risk factors, which could otherwise mask the pure effects of the vitamin D intervention. The participants will be recruited from the same tertiary university hospital as the study 1. The healthy working adults from the institution will be invited to participate via posters/flyers displayed at the site. Potential participants will be invited to contact study coordinating principal investigator if they are interested to take a part in this study. The aim would be to recruit consecutive 75 healthy working adults who express the willingness to participate. Previous allergies to vitamin D supplementation also considered as an exclusion criterion in addition to the exclusion criteria applied in study 1.
Study variables and data collection procedure
Study 1 data collection
Medical history, vitamin D intake and health related quality of life
Detailed medical history (self-reported or Electronic Medical Records/eMR) including medical history and current medication will be collected at the time of study enrolment. Questionnaires and physical assessments will be carried out during the routine clinic visits. Validated dietary vitamin D intake questionnaire and sun exposure questionnaire will be used to collect data regarding daily sun exposure and dietary intake of vitamin D. Data relevant to Health-Related Quality Of Life (HRQoL) will be collected using validated 12-Item Short Form Survey (SF-12) tool. Data relevant to fall incidents and related admissions to health care settings will be collected by reviewing hospital records and self-reported information. Demographic information and physical assessment (height and weight) will be carried out in the clinic at the time of enrolment.
Biochemical parameters
Venous blood samples will be collected for the determination of biochemical parameters (plasma vitamin D (25(OH)D3), Parathyroid Hormone (PTH), plasma markers to determine VED such as ADMA and TSP-1), plasma inflammatory markers (Interleukin-6 (IL-6), Tumor Necrosis Factor α (TNF-α), Tumor Growth Factor β1 (TGF-β1), High-sensitive C-Reactive Protein (hs-CRP)), biochemical markers of frailty/sarcopenia (plasma albumin, Procollagen Type III N-terminal Peptide (P3NP), hemoglobulin), urinary function (including creatinine and Glomerular Function Rate (eGFR) and liver function. The blood samples will be analysed in an accredited clinical laboratory in the research institute (for parameters ADMA, TSP-1, IL-6, TNF-α, TGF-β1, P3NP) and in hospital pathology department (for parameters 25(OH)D3, PTH, hs-CRP, albumin, creatinine, hemoglobulin, eGFR and liver enzymes).
Blood pressure
Resting blood pressure (systolic/diastolic) will be measured after subjects are rested for a minimum of 30 minutes. All measurements will be performed using the same electronic blood pressure machine (RIESTER blood pressure monitor), three times by keeping five- minute time gap between the measurements and the average of the three measurements will be utilized for analysis.
Sarcopenia-related physical frailty
This study will use frailty assessments tools to assess and stratify the frailty status of the participants. Australian modified Karnofsky Performance Scale (AKPS) [33], Edmonton frailty scale [34] and Rockwood Clinical frailty scale [35] will be used as frailty tools in this study. Furthermore, Hand Grip Strength (HGS) will be measured by JAMA digital hand dynamometer (Jamar Plus Digital Hand Dynamometer S.N. 2019030003). The European Working Group on Sarcopenia in Older People (EWGSOP) defined grip strength as a “good simple measure of muscle strength, and it correlates with incident disability for Activities of Daily Living (ADL) and leg strength. Grip strength measured in standard conditions with a well-studied model of a handheld dynamometer with reference populations can be a reliable surrogate for more complicated measures of muscle strength in the lower arms or legs [36].
In this study, the grip strength will be measured in a standing position with the forearm away from the body at the levels of the thigh. The participants will be asked to apply maximum strength of both left and right hands three times. Between each measurement at least 30 seconds of resting interval will be allowed. The grip strength will be defined as the maximally measured grip strength of the three readings of the dominant hand [37].
Cut-off value of HGS to determine sarcopenia
The European Working Group on Sarcopenia in Older People (EWGSOP) recommends the use of normative value (of healthy young adults) rather than other predictive reference populations with cut-off values of muscle strength, at 2 standard deviations below the mean reference value [36]. Currently, reference data for a normative Australian population were unavailable for determining the cut-off values. To overcome this limitation, aged matched healthy population from the study 2 will be selected to determine the sarcopenic status. Their gender specific quintile points (lowest 20%) will be used as reference value.
Cognitive function assessment
Mini-Cog tool (Mini-Cog©; Screening for Cognitive Impairment in Older Adults) will be used for detecting mild cognitive impairment. Permission has been granted for Research Use. The Mini-Cog is a 3-minute instrument that can increase detection of cognitive impairment in older adults. It consists of two components, a 3-item recall test for memory and a simply scored clock drawing test. Mini-Cog has been used in clinical settings for detecting mild cognitive impairments in old adults due to its sensitivity and specificity, and validated against the standardised cognitive assessment tools [38,39]. Table 1 represents the enrolment, screening, and assessment schedule of study 1.
|
|
Enrolment |
Screening |
Close-out |
|
Timepoint |
-30d to 0d |
0d |
90d |
|
Enrolment: |
|||
|
Project Advertisement |
X |
|
|
|
Eligibility screen |
X |
|
|
|
Informed consent |
X |
|
|
|
Appointment Schedule |
X |
|
|
|
Screening and Assessments: |
|||
|
Socio-demographics and situational information |
|
X |
|
|
Medical history and current medication |
|
X |
|
|
Physical Assessment |
|
X |
|
|
pressure (Systolic/diastolic) |
|
X |
|
|
Physical Frailty/sarcopenia assessment |
|
X |
|
|
Cognitive function/frailty assessment |
|
X |
|
|
Biochemical parameters |
|
X |
|
|
Dietary vitamin D intake and sun exposure questionnaire |
|
X |
|
|
Fall incidents and related health care admissions |
|
X |
|
|
Data analysis and dissemination: |
|||
|
Data analysis |
|
|
X |
|
Reporting/outcome dissemination |
|
|
X |
Table 1: The schedule of enrolment, screening and assessments of study 1.
Study 1 will establish the relationship between subclinical Vascular Endothelial Dysfunction (VED) and sarcopenia-related physical frailty. Importantly the study will clarify the independent correlation of inflammatory makers (IL-6, TNF-∞ and TGF-β1) and plasma vitamin D level on the link between VED and frailty syndrome/sarcopenia.
Study 2 intervention and data collection
Intervention
This is a 6-month vitamin D intervention in healthy working adults. The participants who are willing to participate the study will be screened for their eligibility against inclusion and exclusion criteria before enrol into the study. This study will be conducted in 3 study visits (Visit 1: baseline screening, Visit 2: 3 months’ follow-up, Visit 3: 6 months’ follow-up), each visit will take approximately 30-60 min time. Eligible participants will be recruited, and study procedures will be carried out at baseline visit and follow up visits at 3 months and 6 months. The participants will be assessed for the same parameters mentioned in the study 1 above (medical history, vitamin D intake and health related quality of life, biochemical parameters, blood pressure, sarcopenia-related physical frailty and cognitive function assessment). At the baseline visit and the 3-months follow up visit, participants will be given 3 months’ vitamin D supplement (Blackmores™ Vitamin D 1000IU capsules, Blackmores Ltd). The vitamin D capsules will be dispensed by the qualified clinical research pharmacist at hospital pharmacy. The participants will be asked to take 2000 IU (2 capsules) per day (consistent time of the day, preferably in the morning) for 6-month period. However, considering the reverse J-shaped association between vitamin D and cardiovascular risk, vitamin D levels will be re-assessed at 3 months and daily dose will be adjusted according to participants’ 3 months’ plasma 25(OH)D3 level (participant who are low in vitamin D, (25(OH)D3 < 75 nmol/L will continue with the same initial dose, participants at or above sufficient level, 25(OH)D3≥75 nmol/L, will continue with 1000 IU (one capsule) per day). A daily diary will be given to complete, information regarding their vitamin D capsule intake, daily outdoor activities including duration of sun exposure, dietary vitamin D intake/intake of dietary products with vitamin D, any changes to their usual health and any new medication they start and changes to their usual medication regimen. The participants will be asked to return their completed daily diary and product bottles with remaining capsules at the 3 months and 6 months follow up visits. The diary notes and left-over capsule count will be recorded and used for assessing study adherence/compliance. End of study survey will be conducted at the last visit (at 6 months) to gather a feedback from the participants regarding the pilot phase of the intervention, which will support the next step of the study, randomised control intervention. Table 2 represents schedule of enrolment, intervention, and assessments of the study 2.
|
|
Study period |
||||
|
Timepoint Visit/month |
|
Visit 1: (Screening)/1m |
Visit 2: Follow-up/3m |
Visit 3: Follow-up/6m |
Close-out |
|
Assessment window |
-8w to 0w |
0w |
12w |
24w |
24w |
|
Enrolment: |
|||||
|
Project advertisement |
X |
|
|
|
|
|
Eligibility screen |
X |
|
|
|
|
|
Informed consent |
X |
|
|
|
|
|
Appointment Schedule |
X |
|
|
|
|
|
Allocation |
|
X |
|
|
|
|
Intervention: |
|||||
|
Vitamin D supplementation 2000 IU/day |
|
|
|
|
|
|
Vitamin D supplementation 2000 IU/day (if 25(OH)D3 1000 IU/day (if 25(OH)D3≥75 nmol/L) |
|
|
|
|
|
|
Daily diary |
|
|
|
|
|
|
Assessments: |
|||||
|
Socio-demographics and situational information |
|
X |
|
|
|
|
Medical history and current medication |
|
X |
|
|
|
|
Change in medication/medical condition |
|
|
X |
X |
|
|
Physical Assessment (Height/weight) |
|
X |
X |
X |
|
|
Blood pressure (Systolic/diastolic) |
|
X |
X |
X |
|
|
Physical Frailty/sarcopenia assessment |
|
X |
X |
X |
|
|
Cognitive function/frailty assessment |
|
X |
X |
X |
|
|
Biochemical parameters |
|
X |
X |
X |
|
|
Dietary vitamin D intake and sun exposure questionnaire |
|
X |
X |
X |
|
|
Fall incidents and related health care admissions |
|
X |
X |
X |
|
|
Renal function/liver function |
|
X |
X |
X |
|
|
Attendance |
|
X |
X |
X |
|
|
Compliance/Adherence (product count and diary review) |
|
|
X |
X |
|
|
End-of-study survey |
|
|
|
|
X |
|
Data Analysis And Reporting: |
|||||
|
Data analysis |
|
|
X |
|
X |
|
Reporting/outcome dissemination |
|
|
|
|
X |
Table 2: Schedule of enrolment, intervention and assessments of study 2.
Adverse reaction monitoring
No serious adverse reactions are anticipated in the trial, but these will be monitored closely. The participants will be informed to contact study coordinating clinician if they develop any adverse reaction. The adverse reaction/serious adverse reaction form will be utilized if any adverse reaction recorded.
Study adherence monitoring
At 3 months and 6 months visits, participants will be asked to hand over their daily diary and product bottles with leftover capsules. The diaries and leftover capsules will be reviewed to calculate the percentage compliance to the treatment. Positive outcomes of study 2 will provide evidence for introducing vitamin D supplementation in frailty management to correct the initial stages of subclinical CVD. This could be used to prevent development of frailty in elderly before other comorbidities are involved in the process, reducing the impact on the patient’s quality of life and the healthcare system.
Data storage, synthesis and analysis
The collected data will be stored securely on paper based CRFs and de-identified coded data will be stored electronically in Research Electronic Data Capture (REDCap) database, which is customized to have password protection. Only the investigators will get the access to the stored data files and the access is authenticated using staff ID and password. Descriptive analysis will be carried-out to assess the demographic and situational data. Data from study 1, sarcopenic and non-sarcopenic subgroups will be evaluated by t-tests (un-paired)/Mann-Whitney U test, data from study 2 will be analysed by paired t-test/Wilcoxon signed-rank test. The data will be analysed using statistical models of logistic regression and multivariate analysis, to identify the correlations between parameters of sarcopenia-related physical frailty and VED. Linear regression analysis will be carried out to evaluate the relationships between variables and controlled multivariate logistic regression analysis will be used to evaluate the relationship of Vascular Endothelial Dysfunction (VED) and sarcopenia-related physical frailty, independent from patients’ demographic characteristics, medical history, medication and other confounding factors and independent correlation of vitamin D with the inflammatory markers and the physical frailty and sarcopnia. Moreover, multivariate logistic regression analysis also will be carried out to analyse the relationship between cognitive frailty and physical frailty (independent from other confounding factors).
Subject stratification: Subjects will be stratified to “low muscle strength” and “normal” groups, based on their recorded Hand Grip Strength (HGS) values, according to gender and age strata, at the screening stage, for data collection and reporting purposes. The definition and diagnosis of Sarcopenia from the Report of the European Working Group on Sarcopenia in Older People [36,40]. The stratification only will be used for reporting purposes. Data will be analysed in such a way to identify the independent relationships of the parameters.
Study outcome dissemination
Study outcomes will be disseminated through publication according to the SPIRIT statement and will be presented at scientific conferences.
Discussion
Study 1 will establish the relationship between subclinical Vascular Endothelial Dysfunction (VED) and sarcopenia-related physical frailty. Importantly the study will clarify the independent correlation of inflammatory makers (IL-6, TNF-∞ and TGF-β1) and plasma vitamin D level on the link between VED and frailty syndrome/sarcopenia. Positive outcomes of study 2 will provide evidence for introducing vitamin D supplementation in frailty management to correct the initial stages of subclinical CVD. This could be used to prevent development of frailty in elderly before other comorbidities are involved in the process, reducing the impact on the patient’s quality of life and the healthcare system.
This study is carried out in area of high multicultural and rapidly ageing population. Multiculturalism has also been shown to be a significant risk-factor for vitamin D deficiency, frailty and other adverse cardiovascular health outcomes in the area. Aging-associated physical frailty is associated with poor health-outcomes including disability, hospitalization, and mortality with significantly higher healthcare utilization and related economic cost. Therefore, it is essential to implement novel frailty management strategies to improve the quality of life of frail people and reduce the healthcare cost associated with frailty outcomes. The study will investigate the association of vitamin D deficiency and frailty in this highly vulnerable population to better inform prevention strategies. The study findings will be used to implement frailty prevention/controlling strategies at early stages. Success of the study would also form the basis for a larger interventional study to determine the feasibility of early intervention strategies. Thus, results from this study will be aimed at reducing emergency department re-visits, hospital admissions/re-admissions, and longer hospital length of stay through potential new strategies to improve the quality of life of patients with cardiovascular comorbidities thus reducing associated health costs. Positive outcome of the mechanistic study would provide baseline biomedical mechanistic insight for further studies to introduce new pharmacological interventions in frailty.
Authors Contributions
Anjalee T Amarasekera (AA): Study conceptualization, literature review, study design, protocol development and study coordination, data analysis and manuscript writing, corresponding author*
Peter Schwarz (PS): Study conceptualization and design, Literature review and manuscript writing
Dennis Chang (DC): Study conceptualization, study design and manuscript writing
Vlasios Brakoulias (VB): Study conceptualization and protocol development
Timothy C Tan (TT): Study conceptualization, literature review, study design and manuscript writing
Funding Sources
This research was supported by Early/Mid-Career Research Seed Grant of Dr. Anjalee T. Amarasekera, which has been funded by SPHERE (The Sydney Partnership for Health, Education, Research and Enterprise) Cardiac and Vascular Health Clinical Academic Group 2019-2021.
Product supply for intervention phase: Blackmores Ltd Australia (This is industrial research support only, funder does not play any role in study conceptualizing and study design, data collection, analyzing or reporting).
References
- Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, et al. (2015) A reverse J-shaped association between serum 25-hydroxyvitamin D and cardiovascular disease mortality: The CopD study. J Clin Endocrinol Metab 100: 2339-2346.
- Fiscella K, Franks P (2010) Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med 8: 11-18.
- Amarasekera AT, Chang D, Schwarz P, Tan TC (2020) Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing: afaa237.
- Amarasekera AT, Chang D, Schwarz P, Tan TC (2021) Vascular endothelial dysfunction may be an early predictor of physical frailty and sarcopenia: A meta-analysis of available data from observational studies. Exp Gerontol 148: 111260.
- Campos AM, Moura FA, Santos SN, Freitas WM, Sposito AC (2017) Sarcopenia, but not excess weight or increased caloric intake, is associated with coronary subclinical atherosclerosis in the very elderly. Atherosclerosis 258: 138-144.
- Moncada S, Higgs E (2006) Nitric oxide and the vascular endothelium. Handb Exp Pharmacol: 213-254.
- Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, et al. (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99: 3092-3095.
- Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, et al. (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106: 987-992.
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD (2008) Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med 25: 320-325.
- Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, et al. (2009) Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 94: 4023-4030.
- Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, et al. (2004) Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem 279: 35798-35802.
- Talmor Y, Golan E, Benchetrit S, Bernheim J, Klein O, et al. (2008) Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol 294: 1059-1064.
- Wong MS, Delansorne R, Man RY, Svenningsen P, Vanhoutte PM (2010) Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 299: 1226-1234.
- Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, et al. (2002) Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13: 621-629.
- Zhang Q, Zhang M, Wang H, Sun C, Feng Y, et al. (2018) Vitamin D supplementation improves endothelial dysfunction in patients with non-dialysis chronic kidney disease. Int Urol Nephrol 50: 923-927.
- Karakas Y, Sahin G, Urfali FE, Bal C, Degirmenci NA, et al. (2017) Effect of vitamin D supplementation on endothelial dysfunction in hemodialysis patients. Hemodial Int 21: 97-106.
- Kashani HH, Hosseini ES, Nikzad H, Soleimani A, Soleimani M, et al. (2018) The effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic hemodialysis: A randomized, double-blind, placebo-controlled trial. Front Pharmacol 9: 50.
- Farhangi MA, Mesgari-Abbasi M, Hajiluian G, Nameni G, Shahabi P (2017) Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation 40: 1688-1697.
- Hu C, Wu X (2019) Effect of vitamin D supplementation on vascular function and inflammation in patients with chronic kidney disease: A controversial issue. Ther Apher Dial 24: 265-274.
- Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, et al. (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 50: 271-279.
- Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, et al. (2006) Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol 41: 746-752.
- Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, et al. (2003) A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc 51: 291-299.
- Amarasekera AT, Sverdlov AL, Horowitz JD, Ngo DT (2016) Elevated parathyroid hormone predicts high asymmetric dimethylarginine (ADMA) concentrations in obese diabetic patients. Diabetes Metab 42: 378-381.
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, et al. (2002) Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci 57: 326-332.
- Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, et al. (1999) Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation 99: 1141-1146.
- Vallance P, Leiper J (2004) Cardiovascular biology of the asymmetric dimethylarginine: Dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24: 1023-1030.
- Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, et al. (2007) Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 282: 15404-15415.
- Isenberg JS, Frazier WA, Roberts DD (2008) Thrombospondin-1 is a central regulator of nitric oxide signaling in vascular physiology. Cell Mol Life Sci 65: 728-742.
- Miller TW, Isenberg JS, Roberts DD (2010) Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol 159: 1542-1547.
- Amarasekera AT, Assadi-Khansari B, Liu S, Black M, Dymmott G, et al. (2017) Vitamin D supplementation lowers thrombospondin-1 levels and blood pressure in healthy adults. PLoS One 12: 0174435.
- Langa KM, Levine DA (2014) The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 312: 2551-2561.
- Panza F, Lozupone M, Solfrizzi V, Sardone R, Dibello V, et al. (2018) Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J Alzheimers Dis 62: 993-1012.
- Abernethy AP, Shelby-James T, Fazekas BS, Woods D, Currow DC (2005) The Australia-modified Karnofsky Performance Status (AKPS) scale: S revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care 4: 7.
- Jankowska-Pola?ska B, Uchmanowicz B, Kujawska-Danecka H, Nowicka-Sauer K, Chudiak A, et al. (2019) Assessment of frailty syndrome using Edmonton frailty scale in Polish elderly sample. Aging Male 22: 177-186.
- Church S, Rogers E, Rockwood K, Theou O (2020) A scoping review of the Clinical Frailty Scale. BMC Geriatr 20: 393.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412-423.
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, et al. (2011) A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 40: 423-429.
- Carnero-Pardo C, Cruz-Orduña I, Espejo-Martínez B, Martos-Aparicio C, López-Alcalde S, et al. (2013) Utility of the mini-cog for detection of cognitive impairment in primary care: Data from two spanish studies. Int J Alzheimers Dis 2013: 285462.
- Yokomizo JE, Simon SS, de Campos Bottino CM Cognitive screening for dementia in primary care: A systematic review. Int Psychogeriatr 26: 1783-1804.
- Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, et al. (2010) Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc 58: 1721-1726.
Citation: Amarasekera AT, Schwarz P, Chang D, Brakoulias V, Tan TC (2021) Twin D-Frail Study Protocol: Does Vitamin D Link Vascular Endothelial Dysfunction to Sarcopenic Frailty. J Gerontol Geriatr Med 7: 096.
Copyright: © 2021 Amarasekera AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

