
Use of C-Peptide Levels in the Diagnosis and Prevention of Type 1 Diabetes Mellitus as an Immune-Related Adverse Event Secondary to Treatment with Immunotherapy in Solid Tumors
*Corresponding Author(s):
Luis Figuero-PérezDepartment Of Medical Oncology, Instituto De Investigación Biomédica De Salamanca (IBSAL), University Hospital Of Salamanca, Spain
Tel:+34 923 29 11 00,
Email:figuero44@gmail.com
Abstract
Background: Type 1 diabetes mellitus (T1D) as an immune-related adverse event (irAE) is found in 0.5-5% of all patients treated with immunotherapy (IT) and it is associated with life-threatening complications. However, there are no official guidelines that suggest methods for the prevention and monitoring of T1D during treatment with IT. The algorithms described in the literature only assess the fasting blood sugar levels for prevention and monitoring. The objective of this review is to find factors that establish a relationship with the appearance of T1D as an irAE.
Methods: We carried out a clinical review of the cases published in the literature on T1D secondary to IT between 2012 and 2019. Through a statistical analysis, the relevant clinical characteristics will be determined, together with the factors related to the appearance of T1D, which will make it possible to put forward a new algorithm for the prevention and monitoring of T1D during treatment with IT for solid tumors.
Results: Eighty-one cases were reported. Of the reported cases, 90% were associated with Nivolumab or Pembrolizumab. The median age was 67 years (23-84 years), and the patients were predominantly men. The average number of administered doses was 5.3 with a lapse of 14.6 weeks from the administration of the first dose until diagnosis. In 48 cases (59%) the levels of C-peptide were low or undetectable, and the glycosylated hemoglobin (HbA1c) levels were elevated by an average of 8.8% (73mmol/mol). Anti-β-cell antibodies were found in less than half of the patients (44). Diabetic ketoacidosis was observed at diagnosis in 61 patients (75%).
Conclusions: Based on the results of our review, we propose including the levels of C-peptide in the usual protocol for the management of adverse events related to IT.
Keywords
Immunotherapy; C-peptide; Prevention; Diabetes mellitus; Monitoring
INTRODUCTION
Immunotherapy represents one of the fundamental points on which oncological treatments are based and it has supposed a revolution in the treatment of cancer. Immune control points (or “checkpoints”) are cell surface molecules which act as endogenous regulatory immune response and, therefore, are perfect therapeutic targets [1], being possible to act with different drugs that interrupt the inhibitory signals in the T cells, which promote the activation and functioning of these cells and the immune system secondarily, increasing the antitumor response.The two main checkpoints with immune system inhibitory function are the cytotoxic T-lymphocyte antigen 4 (CTLA-4) and the programmed cell death 1 (PD-1) with [2].
CTLA-4 is a receptor which is expressed on the surface of T lymphocytes during the process of activating the immune response. Its main action is inhibitory, regulating homeostasis and peripheral immune tolerance, inhibiting the activation of T lymphocytes through mechanisms of negative signalling and competitive antagonism of the CD28/B7-mediated co-stimulatory pathway [3].
PD-1 is another co-inhibitory membrane receptor expressed in T cells, with the same functions that CTLA-4. The ligands of PD-1 are PD-L1 (B7-H1) and PD-L2 (B7-DC) that are expressed in tissue macrophages and tumour cells [4], which are responsible for the inhibition of T lymphocytes facilitating immune tolerance [3].
Blocking of immune checkpoints (CTLA-4, PD-1 and PD-L1) [5,6] with inhibitory antibodies is accompanied by stimulation and proliferation of activated T lymphocytes against tumour cells. The molecules anti-PD1 (nivolumab and pembrolizumab) [7-15], anti-CTLA4 (ipilimumab) [16,17] and anti-PDL1 (atezolizumab, durvalumab and avelumab) [18-21] are the principals antibodies used in the oncological treatments based on immunotherapy (Table 1).
|
Immune checkpoint inhibitors |
Immunoglobulin type |
Target molecule |
Tumor type |
Autoimmune endocrinopathies |
|
Ipilimumab (MDX-010) |
IgG-1k |
CTLA-4 |
Advanced melanoma Advanced renal cancer |
Hypophysitis (11%) Hypothyroidism (<6%) Hyperthyroidism (2-16%) Adrenalitis (0,3-1,5%) Insulinitis (with nivolumab) Parathyroiditis (with nivolumab) |
|
Pembrolizumab (MK-3475) |
IgG-4k |
PD-1 |
Advanced melanoma and adjuvant Metastatic non-small cell lung cancer Refractory Hodgkin lymphoma Advanced bladder cancer Advanced head and neck cancer
|
Thyroid dysfunction (20-40%) Hypophysitis (<1%) Insulinitis (<1%) |
|
Nivolumab (MDX-1106) |
IgG4 |
PD-1 |
Advanced melanoma and adjuvant Metastatic non-small cell lung cancer Refractory Hodgkin lymphoma Advanced bladder cancer Advanced head and neck cancer Advanced renal cancer |
Thyroid dysfunction (20-40%) Hypophysitis (<1%) Insulinitis (<1%) Parathyroiditis (with nivolumab) |
|
Atezolizumab (MPDL3280A) |
IgG1 |
PD-L1 |
Advanced bladder cancer Metastatic non-small cell lung cancer |
Thyroid dysfunction (<5%) Adrenalitis (0,4%) Hypophysitis (<0,1%) Insulinitis (<1%) |
|
Durvalumab (MEDI4736) |
IgG1 |
PD-L1 |
Locally advanced unresectable non-small cell lung cancer |
Thyroid dysfunction (<7%) Adrenalitis (0,4%) Hypophysitis (<0,1%) Insulinitis (<1%) |
|
Avelumab (MSB0010718C) |
IgG1 |
PD-L1 |
Metastatic Merkel cell carcinoma |
Thyroid disfunction (1-6%) Adrenalitis (0,5%) Insulinitis (<1%) |
Table 1: Main immune checkpoints antibodies with demonstrated clinical effects and associated autoimmune endocrinopathies.
Secondary to these treatments have been noted several side effects, logically related to the activation of the immune system (immune related adverse events “irAEs”). These irAEs are observed in 10-20% of patients with treatments based on immunotherapy [22], being some of theme potentially serious (for example, pneumonitis or colitis).
These adverse events are reporting with the use of appropriate terminology according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 that stratifies the side effects in five grades from lower to higher toxicity.The immune related adverse events are more important and frequent when anti-PD1 molecules are combined with anti-CTLA-4 (approved in advanced melanoma and renal cell cancer, in combination with nivolumab plus ipilimumab), appearing in about 40% of patients. Conversely, in monotherapy, only 10% of patients develop an irAE [22].
The systemic adverse effects most reported in the literature and clinical trials are asthenia, anorexia, fever, arthralgia and headache. Fatigue is the most frequent symptom with the use of checkpoints (reported in 14-15% of patients treated with ipilimumab [16,17,23] and in 35-39% in the combination with nivolumab [17,24-27]). However, only in 4-5% of patients with combination therapy are severe irAEs described (grade III/IV).
Other irAEs frequently described in the literature are the dermatological side effects (pruritus, vitiligo and skin rash), gastrointestinal (colitis), pulmonary (pneumonitis), endocrine (thyroid dysfunction, adrenalitis, insulinitis or hypophysitis) or other less frequent, such as ocular, renal or neurological.
Endocrine irAEs are one of the most important points with the use of this type of drugs, calculating that between 5-10% of the patients develop an endocrinological alteration. The incidence is 9% in treatments based on anti-PD1 or anti-PDL1 molecules, and 15% with anti-CTLA-4 agents and in combination of both molecules [10,17,23]. The most commonly described irAE is thyroiditis (both in its hyperthyroid and hypothyroid forms), with a varying incidence of 5 to 15%, depending on the agent that was used [27]. Other irAEs that have been reported in the literature within the endocrine range are hypophysitis (with an incidence that is likely lower than 5%) [28] and adrenalitis (primary adrenal insufficiency), whose incidence is probably underestimated due to the use of corticosteroids in oncology patients [27].
Insulinitis is an irAE associated with anti-PD1, anti-PDL1 and anti-CTLA-4 agent that has been linked to the development of autoimmune type 1 diabetes mellitus (T1DM). This side effect has been observed in 1-5% of treated patients, appearing both in naïve patients and those previously treated with chemotherapy, as well as in patients with and without a history of autoimmune endocrinopathies [29-31]. The evolution of this adverse effect is variable, having been observed up to one year after the first dose of nivolumab [29-31] and up to four years of the first dose of pembrolizumab [29,32] (Tables 2 and 3). The pathogenic mechanism proposed is the immunological activation derived from PD1 receptor blockade of T lymphocytes with the consequence increase in the immune response. In addition, the production of anti-GAD and anti-IA2 antibodies by B lymphocytes [32], which together destroy pancreatic islets, precipitate the development of T1DM. Some HLA genotypes have been associated with an increased risk of developing these complications, such as the mayor histocompatibility complex (MHC) class II DR3-DQ2 and DR4-DQ8 [32,33]. Secondarily, there is an association of these complications with others of greater severity, such as diabetic ketoacidosis or hyperosmolar coma.
|
Author |
Sex, age |
Cancer |
DKA |
HbA1 |
Drug |
T1DM onset time (nº infusions) |
T1DM onset time (weeks) |
AB |
C-peptide (range) |
Year |
|
Sothornwit et al. |
Female, 52 |
NSCLC |
+ |
7,9% (63mmol/mol) |
Atezolizumab |
5 |
24 |
GAD + |
<0,03 nmol/L (<0,1ng/mL) |
2019 |
|
Yilmaz et al. |
Male, 49 |
Renal cell |
+ |
10,9% (96 mmol/mol) |
Nivolumab |
22 |
41 |
Negative |
0,079nmol/L (non value) |
2019 |
|
Bastin et al. |
Female, 51 |
NSCLC |
+ |
8% (64mmol/mol) |
Nivolumab |
4 |
8 |
GAD + |
<0,010mg/L (non value) |
2019 |
|
Bastin et al. |
Male, 71 |
NSCLC |
- |
13,7% (127 mmol/mol) |
Nivolumab |
U |
28 |
Negative |
2mg/L (non value) |
2019 |
|
Bastin et al. |
Female, 61 |
NSCLC |
+ |
9,3% (78mmol/mol) |
Nivolumab |
4 |
8 |
Negative |
0,04mg/L (non vale) |
2019 |
|
Kotwal et al. |
U |
U |
+ |
8,6% (70mmol/mol) |
Pembrolizumab |
2 |
9 |
Negative |
0,9ng/mL (non value) |
2018 |
|
Kotwal et al. |
U |
U |
+ |
10,7% (93mmol/mol) |
Pembrolizumab |
1 |
10 |
Negative |
<0,1ng/mL (non value) |
2018 |
|
Kotwal et al. |
U |
U |
+ |
7,8% (62mmol/mol) |
Pembrolizumab |
1 |
3 |
GAD + |
<0,1ng/mL (non value |
2018 |
|
Kotwal et al. |
U |
U |
- |
10,5% (91mmol/mol) |
Pembrolizumab |
3 |
19 |
GAD + |
0,3ng/mL (non value |
2018 |
|
Kotwal et al. |
U |
U |
- |
U |
Nivolumab |
4 |
22 |
U |
U |
2018 |
|
Kotwal et al. |
U |
U |
- |
9,7% (82mmol/mol) |
Pembrolizumab + Ipilimumab |
6 |
19 |
U |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
9,7% (82mmol/mol) |
Pembrolizumab |
5 |
31 |
Negative |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
7,8% (61mmol/mol) |
Pembrolizumab |
17 |
38 |
Negative |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
11,3% (99mol/mol) |
Pembrolizumab |
16 |
94 |
U |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
11,2% (97mmol/mol) |
Pembrolizumab |
4 |
12 |
U |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
8,8% (73mmol/mol) |
Pembrolizumab |
2 |
6 |
U |
U |
2018 |
|
Kotwal et al. |
U |
U |
+ |
10,6% (92mmol/mol) |
Pembrolizumab |
4 |
33 |
Negative |
U |
2018 |
|
Maamari et al. |
Male, 47 |
Cardiac angiosarcoma |
+ |
6,4% (43mmol/mol) |
Pembrolizumab |
2 |
6 |
GAD + |
0,1ng/mL (1,1-4,4ng/mL) |
2018 |
|
Zaied et al. |
Male, 70 |
Renal cell |
+ |
8,4% (68mmol/mol) |
Nivolumab |
3 |
6 |
Not measured |
0,44 ng/mL (1,1-4,4 ng/mL) |
2018 |
|
Marchand et al. |
Female, 55 |
NSCLC |
+ |
8,2% (66mmol/mol) |
Nivolumab |
9 |
20 |
Negative |
<0,1 nmol/L (>0,37nmol/L) |
2018 |
|
Marchand et al. |
Female, 72 |
Cutaneous T-cell lymphoma |
- |
11,4% (101 mmol/mol) |
Nivolumab |
3 |
8 |
IA2A + |
1 nmol/L (>0,37 nmol/L) |
2018 |
|
Marchand et al. |
Female, 69 |
NSCLC |
+ |
7,4% (57mmol/mol) |
Durvalumab |
13 |
52 |
Negative |
<0,1 nmol/L (>0,37nmol/L) |
2018 |
|
Marchand et al. |
Female, 83 |
Melanoma |
- |
9,4% (79mmol/mol) |
Pembrolizumab |
4 |
10 |
Negative |
1 nmol/L (>0,37 nmol/L) |
2018 |
|
Marchand et al. |
Female, 65 |
Melanoma |
+ |
8,5% (69mmol/mol) |
Pembrolizumab |
12 |
34 |
Negative |
<0,1 nmol/L (>0,37nmol/L) |
2018 |
|
Marchand et al. |
Male, 65 |
Melanoma |
+ |
7,3% (56mmol/mol) |
Nivolumab + Ipilimumab |
5 |
12 |
Negative |
<0,1 nmol/L (>0,37nmol/L) |
2018 |
|
Stamatouli et al. |
Male, 58 |
SCLC |
- |
U |
Nivolumab |
1 |
U |
Not measured |
U |
2018 |
|
Stamatouli et al. |
Male, 80 |
NSCLC |
- |
U |
Nivolumab |
14 |
U |
Not measured |
U |
2018 |
|
Tzoulis et al. |
Female, 56 |
NSCLC |
+ |
8,2% (66 mmol/mol) |
Nivolumab |
3 |
7 |
GAD + |
Undetectable |
2018 |
|
Tassone et al. |
Male, 42 |
NSCLC |
+ |
6% (42mmol/mol) |
Nivolumab |
4 |
8 |
GAD + |
U |
2018 |
|
Galligan et al. |
Male. 70 |
Melanoma |
+ |
8,9% (73,8mol/mol) |
Pembrolizumab |
2 |
6 |
GAD + |
U |
2018 |
|
Galligan et al. |
Male, 82 |
Squamous cell carcinoma |
- |
8,5% (69,4 mmol/mol) |
Pembrolizumab |
3 |
9 |
Negative |
<0,03 nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 61 |
Melanoma |
+ |
7,7% (60,7 mmol/mol) |
Pembrolizumab |
2 |
8 |
GAD + |
<0,03 nmol/L (non value) |
2018 |
|
Galligan et al. |
Female, 53 |
Melanoma |
+ |
7,2% (55,2 mmol/mol) |
Pembrolizumab |
1 |
3 |
GAD + |
0,04 nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 23 |
Melanoma |
+ |
7,5% (58 mmol/mol) |
Pembrolizumab |
2 |
8 |
GAD + Insulin Ab |
0,11 nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 61 |
Melanoma |
+ |
8,6% (70mmol/mol) |
Pembrolizumab + Ipilimumab |
2 |
6 |
GAD + |
0,12 nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 60 |
Melanoma |
+ |
7,8% (62mmol/mol) |
Pembrolizumab + Ipilimumab |
1 |
5 |
Negative |
0,057 nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 50 |
Melanoma |
+ |
6,4% (43mmol/mol) |
Nivolumab + Ipilimumab |
4 |
12 |
GAD + |
0,01nmol/L (non value) |
2018 |
|
Galligan et al. |
Male, 65 |
Melanoma |
+ |
7,4% (57mmol/mol) |
Pembrolizumab |
1 |
2 days |
Negative |
0,38 nmol/L (non value) |
2018 |
|
Atkins et al. |
Male, 50 |
Squamous cell carcinoma |
+ |
6,4% (43mmol/mol) |
Avelumab + Utomilumab |
U |
U |
Negative |
63 pmol/L (140-830 pmol/L)) |
2017 |
|
Araujo et al. |
Female,73 |
NSCLC |
+ |
7,2% (55 mmol/mol) |
Nivolumab |
2 |
4 |
GAD + |
0,06 ng/mL (<0,1ng/mL) |
2017 |
|
Kapke et al. |
Female, 63 |
Urothelial |
+ |
7,8% (61 mmol/mol) |
Atezolizumab |
9 |
24 |
GAD + |
0,02 ng/mL (1,1-4,4ng/mL) |
2017 |
|
Kapke et al. |
Male, 83 |
Squamous cell carcinoma |
+ |
7,4% (57 mmol/mol) |
Nivolumab |
6 |
12 |
GAD + |
0,32 ng/ml (1,1-4,4ng/mL) |
2017 |
|
Scott et al. |
Male, 58 |
Melanoma |
+ |
6,8% (50 mmol/mol) |
Ipilimumab + Pembrolizumab |
3 |
9 |
Negative |
U |
2017 |
|
Gauci et al. |
Female, 84 |
Melanoma |
+ |
8,6% (70,5 mmol/mol) |
Pembrolizumab |
2 |
6 |
GAD + IA2A + |
U |
2017 |
|
Gauci et al. |
Male, 34 |
Melanoma |
- |
5,4% (35,5 mmol/mol) |
Nivolumab + Ipilimumab |
U |
U |
Negative |
U |
2017 |
|
Gauci et al. |
Male, 73 |
Melanoma |
+ |
8,8% (73 mmol/mol) |
Nivolumab |
3 |
6 |
GAD + ZnT8A + |
0 nmol/L (<0,5ng/mL) |
2017
|
|
Li et al. |
Male, 63 |
NSCLC |
+ |
7,2% (55 mmol/mol) |
Nivolumab |
2 |
4 |
GAD + |
U |
2017 |
|
Leonardi et al. |
Male, 66 |
NSCLC |
+ |
7,6% (60 mmol/mol) |
Pembrolizumab |
3 |
9 |
GAD + |
0,3 ng/mL (1,1-4,4ng/mL) |
2017 |
|
Ishikawa et al. |
Female, 54 |
Melanoma |
U |
7,0% (53 mmol/mol) |
Nivolumab |
16 |
U |
Negative |
<0,1ng/mL (0,8-2,5ng/mL) |
2017 |
|
Alzenaidi et al. |
Male, 46 |
Melanoma |
+ |
8,0% (64 mmol/mol) |
Nivolumab + Ipilimumab |
2 |
U |
GAD + |
0,2ng/mL (0,9-5,5ng/mL) |
2017 |
|
Munakata et al. |
Male, 72 |
Hodgkin´s Lymphoma |
- |
7,3% (56 mmol/mol) |
Nivolumab |
5 |
10 |
Negative |
Reduce |
2017 |
|
Usui et al. |
Female, 62 |
NSCLC |
U |
6,5% (48 mmol/mol) |
Nivolumab |
4 |
12 |
Negative |
U |
2017 |
|
Usui et al. |
Male, 31 |
NSCLC |
+ |
6,4% (46 mmol/mol) |
Nivolumab |
1 |
2 |
GAD + |
<0,03ng/mL (>0,03 ng/mL) |
2017 |
|
Godwin et al. |
Female, 34 |
NSCLC |
+ |
7,1% (54mmol/mol) |
Nivolumab |
2 |
U |
GAD+ |
<0,1ng/mL (0,8-3,85 ng/mL) |
2017 |
|
Thoreau et al. |
Male, 73 |
Melanoma |
+ |
8,5% (69mmol/mol) |
Pembrolizumab |
U |
26 |
Negative |
U |
2017 |
|
Farrell et al. |
Male, 30 |
Melanoma |
+ |
7,4% (57 mmol/mol) |
Pembrolizumab |
U |
U |
Negative |
Undetectable |
2017 |
|
Shah et al. |
Female, 77 |
NSCLC |
+ |
10,2% (89mmol/mol) |
Nivolumab |
1 |
2 |
Negative |
0,81 ng/mL (non value) |
2016 |
|
Hao et al. |
Female, 28 |
Melanoma |
+ |
U |
Nivolumab |
3 |
U |
GAD + |
U |
2016/2017 |
|
Alhusseini et al. |
Male, 56 |
NSCLC |
+ |
8,5% (69 mmol/mol) |
Pembrolizumab + Ipilimumab |
1 |
3 |
GAD + IA2A + |
Undetectable |
2016 |
|
Hofmann et al. |
Male, 40 |
U |
U |
U |
Nivolumab |
U |
6 |
U |
U |
2016 |
|
Hofmann et al. |
Female, 78 |
Melanoma |
+ |
U |
Nivolumab |
2 |
3 |
GAD + |
Low |
2016 |
|
Hofmann et al. |
Female, 70 |
Melanoma |
U |
U |
Nivolumab |
4 |
6 |
Negative |
(140-830 pmol/L) |
2016 |
|
Hofmann et al. |
Female, 58 |
Melanoma |
U |
U |
Pembrolizumab |
1 |
3 |
GAD + |
Low |
2016 |
|
Lowe et al. |
Male, 54 |
Melanoma |
+ |
U |
Nivolumab + Ipilimumab |
3 |
U |
GAD + |
<0,1 ng/mL (non value) |
2016 |
|
Chae et al. |
Male, 76 |
NSCLC |
- |
5,8% (40 mmol/mol) |
Pembrolizumab |
2 |
U |
GAD + IA2A + |
0,81ng/mL (0,9-3,85 ng/mL) |
2016 |
|
Aleksova et al. |
Male, 60 |
Melanoma |
+ |
7,1% (54 mmol/mol) |
Pembrolizumab |
2 |
5 |
Negative |
57pmol/L (300-2350 pmol/L) |
2016 |
|
Okamoto et al. |
Female, 55 |
Melanoma |
+ |
7,0% (53 mmol/mol) |
Nivolumab |
U |
52 |
Negative |
<0,1ng/mL (0,61-2,09ng/mL) |
2016 |
|
Miyoshi et al. |
Female, 66 |
Melanoma |
+ |
10,7% (93mmol/mol) |
Pembrolizumab |
9 |
U |
Negative |
U |
2016 |
|
Humayun, et al. |
Male, 55 |
Melanoma |
+ |
10,7% (93 mmol/mol) |
Pembrolizumab |
9 |
U |
Negative |
U |
2016 |
|
Teramoto et al. |
Female, 63 |
Melanoma |
+ |
8,9% (74 mmol/mol) |
Nivolumab |
8 |
U |
Negative |
0,08ng/mL (non value) |
2016 |
|
Hansen et al. |
Male, 58 |
Melanoma |
U |
9,7% (83 mmol/mol) |
Pembrolizumab |
17 |
52 |
GAD + |
2,4ng/mL |
2016 |
|
Hughes et al. |
Male, 58 |
SCLC |
+ |
9,7% (83 mmol/mol) |
Nivolumab |
U |
1 |
GAD + |
<0,1ng/dL |
2015 |
|
Hughes et al. |
Male, 63 |
Renal cell |
- |
8,2% (66 mmol/mol) |
Nivolumab |
U |
16 |
GAD + ICA + IAA + |
1,3ng/dL (1,1-4-4ng/mL) |
2015 |
|
Hughes et al. |
Female, 83 |
NSCLC |
+ |
7,7% (61 mmol/mol) |
Nivolumab |
U |
4 |
GAD + |
<0,1ng/dL (1,1-44ng/mL) |
2015 |
|
Hughes et al. |
Female, 55 |
Melanoma |
+ |
6,9% (52 mmol/mol) |
Nivolumab |
U |
20 |
U |
<0,1ng/dL (1,1-4,4ng/mL) |
2015 |
|
Hughes et al. |
Female, 64 |
Melanoma |
+ |
7,4% (57mmol/mol) |
Pembrolizumab |
U |
4 |
Negative |
0,5ng/mL (1,1-4,4ng/mL) |
2015 |
|
Martin-Liberal et al. |
Female, 54 |
Melanoma |
+ |
U |
Pembrolizumab |
3 |
U |
GAD + |
U |
2015 |
|
Mellati et al. |
Female, 66 |
Squamous cell carcinoma |
+ |
9,4% (79mmol/mol) |
Anti-PD1 |
3 |
7 |
GAD + |
<0,1 ng/mL |
2015 |
|
Mellati et al. |
Male, 70 |
NSCLC |
+ |
9,8% (84 mmol/mol) |
Anti-PD1 |
5 |
15 |
Negative |
0,3ng/mL (1,0-7,1ng/mL) |
2015 |
|
Gaudy et al. |
Female, 44 |
Melanoma |
+ |
6,8% (52 mmol/mol) |
Pembrolizumab |
2 |
U |
Negative |
Undetectable |
2015 |
|
Brahmer et al. |
U |
U |
U |
U |
Anti-PD1 |
U |
U |
U |
U |
2012 |
DKA=Diabetic ketoacidosis; HbA1c=Glycated haemoglobin; AB=Antibody; U=Unknown; NSCLC=Non small cell lung cancer; GAD=Glutamic acid decarboxylase antibody; IA2A=Islet antigen 2 antibody; ZnT8A=Zinc transporter 8 antibody; IAA=Insulin antibody; ICA=Islet cell antibody; SCLC=Small cell lung cancer.
Table 2: Reported cases in the literature.
|
Reported cases: |
81 |
|
Drug |
|
|
· Nivolumab |
32 |
|
· Pembrolizumab |
32 |
|
· Anti-PD1/PDL1 + Anti-CTLA4 |
11 |
|
· Anti-PD1 |
67 |
|
· Anti-PDL1 |
3 |
|
Demographic data |
|
|
· Sex (Female/Male/Unknown) |
30/38/13 |
|
· Age (years) |
67 (23-84) |
|
Presentation |
|
|
· DKA |
61/81 |
|
· HbA1c |
8,8% (73mmol/mol) |
|
· Peptide-C undetectable or low |
48/81 |
|
· Beta cell antibodies + |
36/81 |
|
Time of diagnosis after start of immunotherapy |
|
|
· Number of doses |
5,3 |
|
· Onset in weeks |
14,6 |
DKA=Diabetic ketoacidosis; HbA1c=Glycated haemoglobin.
Table 3: Characteristics of reported patients with T1DM secondary to treatments based on immunotherapy.
The treatment of the vast majority of irAEs is based on the use of corticosteroids, which are routinely used in serious complications (grade III/IV) and in some case mild (grade I/II). In endocrine irAEs, the use of corticosteroids is extended for cases of pituitary and adrenal alterations, as well as severe thyroid dysfunctions. On the other hand, in case of autoimmune T1DM secondary to immunotherapy agents, the use of glucocorticoids is not widespread, and the treatment is based on hormone replacement with insulin, although corticosteroids have not been demonstrated as an agent that can reverse the process of pancreatic destruction or improving symptomatology in these patients [34].
We have performed a review that has allowed us to obtain a profile of patients who present with autoimmune diabetes secondary to checkpoint inhibitors. With this knowledge we have developed an algorithm that can help diagnose these adverse effects early and avoid acute complications such as ketoacidosis or chronic.
MATERIALS AND METHODS
A clinical review of the literature on immunotherapy and its side effects at the endocrine level has been carried out, including a search of online databases and bibliographic reviews. Special interest has been shown in ambulatory endocrine monitoring protocols in patients in treatments based on these agents, as well as reports of clinical cases in patients in whom related diabetic complications have been found.
The incidence of these side effects has been registered by consulting the clinical trials published about these drugs and the technical data sheets of the medicines of the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Based on the above, several figure and tables have been made, based on a summary of what is found in the literature and added to the knowledge that we propose to update on the monitoring algorithm of this type of drugs, that includes more comprehensive the monitoring of endocrine irAE at the pancreatic level.
RESULTS
As note in the introductory section, side effects with oncological treatments based on inhibitory molecules of immune checkpoints are frequently. Endocrine irAEs occur in 5-10% of patients (0,5-5% pancreatic), and can become in many cases severe [16,17,23]. These include alterations of fasting basal blood glucose or development of T1DM.Up to now in literature, the T1DM cases secondary to anti-PD1 treatment are more frequent according to the literature with nivolumab (0,9%) than with pembrolizumab (0,2%). The mean onset of post-treatment endocrine pancreatic complications may range from 15 days to 4 years (with an average of 4,4 months) [23,35-38]. As for anti-PDL1 agents (atezolizumab) in a study of 1.978 patients with non-small cell lung cancer, a total of three developed T1DM (Spira et al., 2015) [39], and two cases of T1DM treated with avelumab have been described in the literature (0,1%, 2/1.738) in patients with advanced gastric cancer (Yamada et al., 2015) [40]. In a phase 1/2 study conducted on 198 patients with non-small cell lung cancer treated with durvalumab, one patient developed T1DM secondary to treatment (Rizvi et al., 2015) [41].
In the analysis of the results of our study, it was observed that in 90% (72/81) of the patients, the development of T1D was secondary to anti-PD1 agents. Monotherapy anti-PDL1 molecules accounted for 3.7% (3/81) of the cases. The mean age at diagnosis was 67 years (23-84), with a predominance of women over men (38 patients over 30,13 patients did not describe the age). The mean dose of drug administered was 5.3 doses with a mean time of onset of endocrine complications of 14.6 weeks (time from first dose administration until diagnosis).
The most frequent clinical debut of patients with T1D secondary to immunotherapy was diabetic ketoacidosis in 75% of patients (61/81). At diagnosis, 59% (48/81) of the patients had a low or undetectable C-peptide value with an average glycosylated haemoglobin (HbA1c) of 8.8% (73mmol/mol). Anti-pancreatic cell antibodies were increased in 44% (36/81) of the patients, of which the most frequently found altered antibody, was anti-GAD (42%, 34/81).
DISCUSSION
The T1DM induced by immunotherapy treatments has its particularities due to the rapid destruction of pancreatic islets. The current bibliography and our results show repeated cases of diabetic debuts abruptly and with a difficult control of glycaemic levels, given the complete absence of pancreatic reserve (levels of peptide-C at diagnosis practically undetectable) at the time of diagnosis [35-37]. However, despite the above, glycaemic levels in the weeks prior to diabetic onset were normal, which gives us an image of the rapid development of hyperglycaemia and associated complications [35,42]. All this translates into a high risk of developing adverse effects with potential vital consequences, such as possible diabetic ketoacidosis [35,37].
During the treatment of T1DM secondary to immunotherapy, a multidisciplinary approach that includes both the oncologist and the endocrinologist is fundamental [43-46], given that the use of corticosteroid therapy has not been shown to be able to stop the destruction of pancreatic islets [34]. Therefore, the treatment is based on the use of insulin and diabetological control similar to the idiopathic pathology. It must be knownthat both onset of T1DM and its diabetic complications is not a requirement for the withdrawal of oncological treatment, and a benefit-risk assessment must be made [27,47-49], and in case of maintaining the treatment should be strictly monitored the patient.
All pathologies developed secondary to treatments with immune checkpoints present a particular development compared to how they would do in their idiopathic form. During the ambulatory monitoring of this type of patient there is a series of analytical markers that are determined routinely prior to the administration of each dose of drug. However, there are no validated protocols that show a sequence in the follow-up and early detection of endocrine irAE.
Endocrine monitoring consists of several points that encompass the thyroid, pituitary, adrenal and pancreatic function, and the determination of sexual hormones is optional and is left to the judgement of the doctor treating the patient based on their clinical assessment [46]. The pancreatic endocrine irAEs do not count in the current bibliography with a protocolized follow-up, being only present the assessment of the basal fasting glycaemia within the analytical measurements of the patients. Figure 1 shows a screening and monitoring endocrine protocol summary of the current literature found.
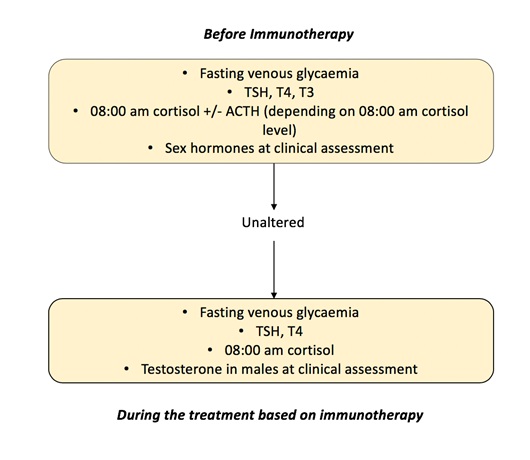 Figure 1: Screening and monitoring of patients under immunotherapy treatment.Summary of protocols in the literature.
Figure 1: Screening and monitoring of patients under immunotherapy treatment.Summary of protocols in the literature.
The singular development of T1DM in patients with treatments based on immunotherapy would require an extension of the analytical monitoring, given the speed of establishment of the clinical picture with its complications. The root of the pathology is in the rapid destruction of the pancreatic islets with the consequent decrease in the pancreatic reserve and consequently in the secretion of insulin [35,37]. All the clinical pictures described in the literature of diabetic ketoacidosis secondary to anti-PD1 or anti-PDL1 agents have in common the scarce pancreatic reserve of patients at the time of diagnosis and the normality in the glycaemia figures in the weeks prior to diagnosis.
There are certain HLA genotypes that have been associated with a greater predisposition to suffer from these conditions [33,43], however, they have not shown the necessary predictive value to be useful in the monitoring of these patients. It has been suggested in the literature that the seroconversion of anti-GAD, anti-IA2 and anti-ZnT8 antibodies secondary to immunotherapy treatments could explain certain points of the physiopathology of the clinical picture [33], this hypothesis not being demonstrated (finding in our study that less than half of the patients had high antibodies at the time of diagnosis). Therefore, serial measurement of antibodies does not fall within the analytical follow-up of these patients, except in the guided exploration of T1DM secondary to immunotherapy when clinical symptoms have been suspected. Despite this, it is possible that the great majority of patients who develop these antibodies during the treatment are in the preclinical phase, and therefore there is another sum of factors that precipitate these clinical pictures (Kotwal et al., 2018) [44,45]. Due to this, starting analytic monitoring in those patients with the serial measurement of T1DM-related antibodies does not currently have any scientific basis.
Consistent with the foregoing, in this review we propose to include the peptide C ofr the screening and monitoring patients under treatment with anti-CTLA4, anti-PD1 and anti-PDL1 agents and their combinations, in which the previous measurement is included to the treatment and then serially every 1 month of the levels of peptide-C [46]. By performing analytical determinations of the previous markers, early detection of pancreatic destruction is possible, making it possible to implement preventive measures or treatment if is necessary prior to the onset of T1DM and its complications. Appearances of endocrine irAEs after the withdrawal of immunotherapy treatment have been described, which gives insight into the pathophysiological changes that occurred during the treatment and the subsequent consequences of these.Due to the above, it would not be clarified until when the analytical markers should continue to be determined after the end of treatment [46-50].
As shown in Tables 2 and 3, most of the clinical cases described present low or virtually inexistent levels of C-peptide on diagnosis. Consequently, it is necessary to measure the serum levels not only after starting the treatment, but also prior to the administration of the first dose in order to know the basal levels of C-peptide presented by the patient.
There are no predetermined levels below which it is necessary to start a diabetes study in patients treated with immunotherapy. The acquired trend of C-peptide levels during the consecutive measurements would really determine the need to analyze other serum levels (autoantibodies or ketone bodies in urine). Logically, decreased levels from the beginning would make it necessary to carry out a complete diabetes study before starting the treatment with immunotherapy.
As Table 3 shows, the diagnosis of T1DM or the appearance of associated complications is not generally observed until the administration of the fifth dose. Therefore, diabetic complications appear after the fourth month of treatment. In the algorithm presented in our review we suggest that C-peptide should be determined during the immunotherapy treatment and prior to the treatment, every 1 month, to get an early diagnosis. The implementation of a multidisciplinary management protocol for these patients from the Departments of Oncology and Endocrinology is essential in order to allow Oncology specialists to reach an adequate interpretation of the C-peptide and HbA1c levels.
CONCLUSION
The endocrine irAEs are one of the points most associated with immunotherapy treatments. The autoimmune T1DM secondary to the anti-CTLA4, anti-PD1 and anti-PDL1 agents presents a different development from the idiopathic form, with an appearance of the clinical picture faster and associated with a greater number of complications. The screening and follow-up after the start of the treatment by means of the analytical determination of C-peptide every 1 month of treatment will allow the early detection of the development of T1DM and the decrease of secondary clinical events, avoiding possible severity symptoms, increasing the quality of life of patients and improving the performance of oncological treatments. More studies will be necessary to help to specify the measures for screening and monitoring of patients in treatments based on immunotherapy.
REFERENCES
- Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348: 56-61.
- Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint in cancer therapy. J Clin Oncol 33: 1974-1982.
- Ott PA, Hodi FS, Robert C (2013) CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 19: 5300-5309.
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162-174.
- Peterson JJ, Steele Moses SK (2016) Update on new therapies with immune checkpoint inhibitors. Clin J Oncol Nurs 4: 405-410.
- Ma W, Gilligan BM, Yuan J, Li T (2016) Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol 27: 47.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, et al. (2015) Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372: 2521-2532.
- Reck M, Rodríguez Abreu D, Robinson AG, Hui R, Csöszi T, et al. (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375: 1823-1833.
- Paz Ares L, Luft A, Vicente D, Tafreshi A, Gümüs M, et al. (2018) Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 379: 2040-2051.
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, et al. (2017) Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 376: 1015-1026.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, et al. (2019) Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 380: 1116-1127.
- Borghaei H, Paz Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373: 1627-1639.
- Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, et al. (2019) First-line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol 37: 992-1000.
- Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, et al. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 378: 1277-1290.
- Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas D, et al. (2016) Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375: 1856-1867.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 8: 711-723.
- Eggermont AM, Sileni VC, Grob JJ, Dummer R, Wolchok JD, et al. (2016) Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375: 1845-1855.
- Reck M, Mok Ts, Nishio M, Jotte RM, Cappuzzo F, et al. (2019) Atezolizumab plus bevacizumab and chemotherapy in non-small-cell cancer (Impower150): Key subgroup analyses of patients with EGFRmutations or baseline liver metastases in a randomized, open-label phase 3 trial. Lancet Respir Med 7: 387-401.
- Horn L, Mansfield AS, Szcezésna A, Havel L, Krzakowski M, et al. (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379: 2220-2229.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, et al. (2017) Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377: 1919-1929.
- D`Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, et al. (2018) Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastasic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol 4: e180077.
- Spain L, Diem S, Larkin J (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51-60.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, et al. (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372: 320-330.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, et al. (2015) Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803-1813
- Larkin J, Chiarion Sileni V, González R, Grob JJ, Cowey L, et al. (2015) Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23-24.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, et al. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372: 2006-2017.
- Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, et al. (2013) Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98: 1361-1375.
- Juszczak A, Gupta A, Karavitaki, Middleton MR, Grossman AB (2012) Ipilimumab: A novel immunomodulating therapy causing autoimmune hypophysitis: A case report and review. Eur J Endocrinol 167: 1-5.
- Hughes J, Vudattu N, Sznol M, Gettinger, Kluger H, et al. (2015) Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 4: e55-e57.
- Hoffmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, et al. (2016) Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 60: 190-209.
- Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, et al. (2016) Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig 7: 915-918.
- Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, et al. (2017) A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother 66: 25-32.
- Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, et al. (2016) Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer 4: 89.
- Aleksova J, Lau PKH, Soldatos G (2016) Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep.
- Godwin JM, Jaggi S, Sirisena I, Sharda P, Rao AD, et al. (2017) Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastasic lung cancer. Journal for ImmunoTherapy of Cancer 5: 40.
- Mellati M, Eaton KD, Worrell BM, Hagopian WA, Martins R, et al. (2015) Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care 38: e137-e138.
- Tzoulis P, Corbett RW, Ponnampalam S, Baker E, Heaton D, et al. (2018) Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol Diabetes Metab Case Rep.
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, et al. (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomized dose-comparison cohort of a phase 1 trial. Lancet Oncol 384: 1109-1117.
- Spira AI, Park K, Maziéres J, Vansteenkiste JF, Rittmeyer A, et al. (2015) Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 33: 15.
- Yamada Y, Nishina T, Iwasa S, Shitara K, Muro K, et al. (2015) A phase I dose expansión trial of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric cancer. J Clin Oncol.
- Rizvi NA, Brahmer JR, Ou SHI, Segal NH, Khleif S, et al. (2015) Safety and clinical activity of MEDI4735, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 366: 2455-2465.
- Sothornwit J, Phunmanee A, Pongchaiyakul C (2019) Atezolizumab-Induced Autoimmune Diabetes in a Patient with Metastasic Lung Cancer. Front Endocrinol (Lausanne)10: 352.
- Gaudy C, Clévy C, Monestier S, Dubois N, Préau Y, et al. (2015) Anti-PD1 Pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 38: e182-e183.
- Kotwal A, Haddox C, Block M, Kudva YC (2019) Immune checkpoint inhibitors: An emerging cause of insulin-dependent diabetes. BMJ Open Diab Res Care 7: e000591.
- Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJ, et al. (2004) Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53: 384-392.
- Hernández AO, Cornejo RA, Pérez LF, Tocino MRV, Martín EE, et al. (2020) Use of glycosylated hemoglobin and C-peptide levels in the diagnosis and prevention of type 1 diabetes mellitus as an immune-related adverse events secondary to treatment with immunotherapy in solid tumors. J Clin Oncol 38: 5.
- Schadendorf D, Dummer R, Hauschild A, Robert C, Hamid O, et al. (2016) Health-related quality of life in the randomized KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer 67: 46-54.
- Ryder M, Callahan M, Postow A, Wolchok J, Fagin JA (2014) Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer 21: 371-381.
- Rahman OA, Halawani HE, Fouad M (2016) Risk of endocrine complications in cancer patients treated with immune chek point inhibitors: A meta-analysis. Future Oncol 12: 413-425.
- Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, et al. (2019) French endocrine society guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer 26: G1-G18.
Citation: Olivares-Hernández A, Cornejo RAE, Figuero-Perez L, Tocino MRV, Sarmiento RG, et al. (2020) Use of C-Peptide Levels in the Diagnosis and Prevention of Type 1 Diabetes Mellitus as an Immune-Related Adverse Event Secondary to Treatment with Immunotherapy in Solid Tumors. J Clin Immunol Immunother 6: 034.
Copyright: © 2020 Alejandro Olivares-Hernández, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

