
Use of Curcumin with Tyrosine Kinase Inhibitors in EGFR-mutant Non-Small Cell Lung Cancer. A Phase I prospective Cohort Trial
*Corresponding Author(s):
Goulnar KasymjanovaJewish General Hospital, Peter Brojde Lung Cancer Centre, Montreal, Canada
Tel:+1 514340-82222, 24312
Email:gkasymja@jgh.mcgill.ca
Abstract
Introduction: Curcumin has been shown to modulate many of the known pathways causing resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGFR-TKIs) in pre-clinical studies.
Methods: This is a phase 1 open prospective cohort study focused on feasibility and safety of combination of enhanced bioavailable formulation of curcumin (CURCUViva TM at 80 mg/ 1 capsule per day for 60 days) in conjunction with an EGFR-TKI in patients with metastatic NSCLC.
Results: 55 patients were approached, 14 (25%) declined to participate in the study: 8 were living far from the hospital and 6 had low interest in curcumin. Of the 41/55 (75%) patients willing to participate, 20 were enrolled; the remaining 21 were already taking another supplement. One excluded before commencing curcumin due to abnormal liver enzymes. 16/19 (84%) completed intended treatment and 86% of them adhered to prescribed treatment. Adverse events profiles were mild, pre-existing and related to TKIs. The CRP level reduced significantly (p<0.05) during the treatment from 15.5 g/L at baseline to 7.38 at week 2, and increased shortly after discontinuation of curcumin. There was significant improvement in lung cancer symptoms (FACT-LCS; p=0.006) and Trial Outcome Index (FACT-TOI; p=0.011) as well as in the Emotional Well-Being subscale.
Conclusion: Our study is the first prospective clinical study using combination of curcumin and EGFR-TKIs in metastatic lung cancer patients. The future randomized larger-scale clinical trials using this combination is feasible and safe. RCT will seek to assess the potential effects on survival and response to TKIs.
Keywords
Curcumin; EGFR mutations; Lung cancer; Safety; TKI
Introduction
Lung cancer is the leading cause of cancer mortality among both men and women in North America. In 2019 alone, there was an estimated 228,150 new cases of lung cancer in the United States, accounting for 142,670 deaths nation-wide [1]. Approximately 80-85% of lung cancers are classified as Non-Small-Cell Lung Cancer (NSCLC), with histological subcategories including adenocarcinoma, squamous cell carcinoma and large cell carcinoma. Approximately 75% of patients with NSCLC present with advanced disease at the time of diagnosis [2], with an estimated overall survival rate of 70% at 12 months [3]. For those patients whose advanced NSCLC harbors an Epidermal Growth Factor Receptor (EGFR) mutation (10% in the United States), Targeted therapy with Tyrosine Kinase Inhibitors (EGFR-TKIs) is considered the standard of care, conferring as much as two- to four-fold greater progression-free survival and increased quality of life compared to cytotoxic chemotherapy [4-8].
Many patients with lung cancer also opt to use natural products concurrently with conventional treatment. One such example is curcumin, or diferuloylmethane, derived from the plant turmeric and commonly consumed throughout the world as the main ingredient in the popular spice known as curry. Curcumin’s anti-inflammatory and anticancer properties have repeatedly been demonstrated both in vitro and in animal models [9-11], including its ability to slow the progression of lung cancer cell lines [12,13]. Though the exact mechanisms through which it modulates gene expression have not yet been fully elucidated, its primary target is thought to be Nuclear Factor Kappa B (NF-κB) [14-16], an upstream signaling molecule linked to lung cancer oncogenes is and cell proliferation [17-19]. Recent in vitro data suggest that curcumin also holds the potential to modulate many of the known resistance pathways to EGFR-TKIs by dose-dependently suppressing EGFR expression [20-22], reducing transactivation of the MET oncogene [23], and reducing inflammation in the tumor microenvironment through selective inhibition of TGF-β [24], TNF-α and NFκB [25]. Synergistic action with gefitinib has also been observed in EGFR-TKI resistant cell lines in mice model of NSCLC [26].
Curcumin’s safety profile is supported by a growing body of evidence [27-29], particularly among cancer populations, in which its potential role as an adjuvant therapy to conventional chemotherapeutic agents is increasingly studied [30-33]. However, despite these promising findings, no studies have yet assessed the safety or efficacy of using curcumin in a lung cancer population in combination with EGFR-TKIs. In addition, curcumin has shown low solubility, poor absorption and rapid breakdown within the body (with prominent first-pass metabolism), resulting in poor oral bioavailability and minimal therapeutic effect. This has led to the development of multiple approaches to increase serum and tissue concentrations, including the use of adjuvant molecules, liposomal particles, phospholipid complexes, curcumin nanoparticles and structural changes [34-36].
The purpose of the current study is to determine the feasibility, and safety of using an enhanced oral bioavailable formulation of curcumin alongside an EGFR-TKI in patients with advanced NSCLC, in addition to assessing its anti-inflammatory properties and impact on health-related quality of life.
Materials And Methods
Study drug
For the purposes of this study, we used CURCUVivaTM lipid-enhanced curcumin capsules from Advanced Orthomolecular Research (AOR); NPN 80027414, approved and licensed by Health Canada. Each capsule contains 80 mg of plant-based Longvida Optimized Curcumin (Turmeric Extract 25:1). This formulation, consisting of Solid Lipid Curcumin Particles (SLCP), is shown to significantly increase oral bioavailability over curcumin alone [37,38]. The study was approved by the Jewish General Hospital Research Ethics committee (Protocol: JGH-14-149, date of approval Feb 10, 2015) and authorized by Health Canada (NCT02321293)
|
Substance (INN): |
CurcuVIVA™ |
|
NPN number |
80027414 |
|
Pharmaceutical form: |
Capsules |
|
Source: |
AOR |
|
Unit strength: |
Turmeric Extract 25:1 348 mg (containing 80mg Longvida® Optimized Curcumin) |
|
Daily dose: |
80 mg with an option to reduce the dose based on individual tolerability (see Section 4.5). |
|
Duration of use: |
Continuous daily dosing for 60 days. |
|
Route of administration: |
Oral (swallowed) |
|
Posology: |
Once daily |
Patients with a histological diagnosis of advanced/metastatic NSCLC cancer and documented presence of sensitive EGFR mutation on first-line chemotherapy were recruited at the Peter Brojde Lung Cancer Centre, Jewish General Hospital to receive standard-of-care EGFR-TKI therapy (at that time: gefitinib or erlitinib or afatinib) and CURCUViva TM at 80 mg/ 1 capsule per day for 60 days. Patients then stopped curcumin and continued their EGFR-TKI with monitoring for two additional months. Safety and quality of life monitoring performed at week 2, week 4, week 8 and 2 months after completion of curcumin treatment.
Patient eligibility
Inclusion criteria for the study were:
- Recent diagnosis or ongoing treatment for histologically-confirmed advanced EGFR-mutant NSCLC, defined as unrespectable stage 3A, 3B or 4
- EGFR mutation analysis performed using the Roche COBAS EGFR v2 qPCR test, with documentation of any positive results, prior to enrollment into the trial
- At least one month of EGFR-TKI therapy prior to starting curcumin
- ECOG performance Score 0-3
- ≥18 years of age
Exclusion criteria included:
- Symptomatic brain metastases
- Other concurrent investigational agents
- Other non-vitamin or mineral natural health products (including Chinese herbs)
- Inability to provide consent, and pregnancy
Sample size
There is no consensus on the required sample size for pilot feasibility studies, and recommendations vary from 10 to 60 participants [39,40]. The main objective of the study is estimating the rate (proportion) of eligible patients who are willing to participate, and who comply with their allocated intervention.
With the sample size of 20 we will be able to estimate the participation rate of 50% within 95% confidence interval of +/-10%. In general, every year, 20 of new diagnosed stage 4 NSCLC patients are found to have an EGFR mutation. Considering estimated 30% drop-out and refusal rate, we determined the study period of 1.5 years.
Outcome measures
Feasibility was assessed by:
- Willingness of patients to participate: number of enrolled/number of approached patients
- Completion rate: number of patients who finished the 60-day course
- Adherence/Compliance rate: total number of capsules consumed/capsules dispensed
- Questionnaires rate: number of completed questionnaires/total number of questionnaires
Quality of life (QOL) was assessed via the Functional Assessment of Cancer Therapy - Lung (FACT-L) questionnaire38 at baseline directly before starting curcumin, then after 2, 4 and 8 weeks of concurrent curcumin and EGFR-TKI treatment.
Hematological toxicity was assessed by: blood counts, serum chemistries and C-Reactive Protein (CRP) levels at baseline, weeks 2, 4, 8 and 16 weeks.
Adverse events were assessed during each visit. In addition, patients recorded additional new symptoms in their diary throughout the study and these were reviewed to identify new or worsening of existing symptoms. The severity of symptoms or any other potential adverse reactions was determined with reference to the National Cancer Institute Common Terminology Criteria v 4.0.
Statistical analysis
All analyses were based on the Treated Set (TS), which includes all patients who were dispensed trial medication and had taken at least one dose of investigational treatment. Data was analyzed using IBM SPSS statistics 20 software (SPSS Inc., Chicago, Illinois, USA).
Given the phase 1 nature of the study and to ensure safety of patients, an interim analysis was performed after first 3 patients had completed 2 months of treatment with concurrent EGFR-TKI and curcumin and had been followed for an additional month with no clinical evidence of serious adverse events or disease progression. The prespecified decision rule was to stop the study if 1/3 patients develop serious adverse events unrelated to EGFR-TKIs. Following the interim analysis, he study was expanded to the intended 20 participants.
Demographic variables are summarized as mean (range) for continuous variables and n (%) for categorical variables.
Outcome variables were defined as:
- Change in Quality of Life (QOL) was defined as the difference between the score at different time points, expressed as a mean (standard deviation). In addition, the clinically meaningful change (CMC) was defined as a 6-point cut-off for the Trial Outcome Index (TOI) and 2 points for the Lung Cancer Subscale (LCS) of the FACT-L [41]. TOI was calculated as the sum of the Physical Well-Being (PWB), Functional Well-Being (FWB) and LCS. Comparisons of repeat measures were made using ANOVA; c2 test and paired sample t-test where appropriate. A p-value of ≤05 was considered statistically significant.
- Changes in blood parameters were analyzed using the paired t-test.
Safety was based on all reported symptoms and/or adverse reactions. Symptoms and/or adverse reactions present at baseline were considered non-related to curcumin.
Results
Patient characteristics and interest in the study
A total of 55 patients were approached to recruit the enrolment target of 20 between July 25, 2015 and April 14, 2017. Fourteen patients declined to participate in the study: 8/14 were living far from the hospital and 6 had low interest in curcumin. Of the 41/55 (75%) patients willing to participate, 20 were enrolled in the study; the remaining 21 were already taking another form of natural supplement or receiving other investigational drugs. One patient was withdrawn before commencing curcumin due to abnormal liver enzymes and CRP level, and was excluded from analysis (Figure 1).
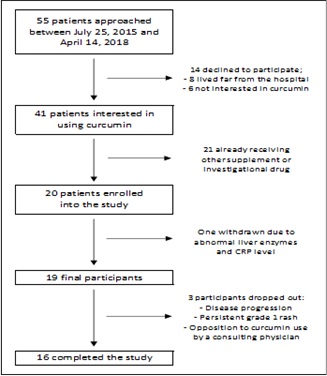 Figure 1: Study flow chart.
Figure 1: Study flow chart.
16/19 participants completed the 60-day course of curcumin. Thus, the overall completion rate was 84%. Three patients dropped out of the study for the following reasons: disease progression, opposition to curcumin use by a treating physician, and a persistent grade 1 rash, likely related to TKI use. The adherence rate was 86 % (984/1140 capsules) (Figure 2).
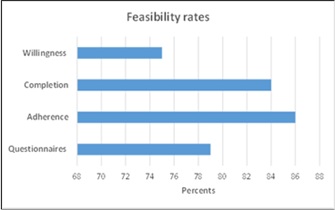
Figure 2: Feasibility assessment.
Primary clinical data for the TS population are summarized in table 1. The mean age was 69, range 46 – 85. Majority of patients (79%) were Caucasian and 21% were Asian. The main metastatic sites were contralateral lungs, pleura, brain and bones. Of note, gefitinib, erlotinib and afatinib were the three EGFR-TKIs used by the studied population, as these were standard of care at the time of patient recruitment. The most common TKI used in the study populations was gefitinib (75% of patients).
|
Variable |
N (%) |
|
Ethnicity |
|
|
Caucasian |
15 (79) |
|
Asian |
4 (21) |
|
Gender |
|
|
Male |
5 (26) |
|
Female |
14 (74) |
|
Age |
|
|
Mean |
69 |
|
Range |
46 - 85 |
|
Site of metastasis |
|
|
Contralateral lung |
7 (28) |
|
Pleura |
6 (24) |
|
Brain (asymptomatic) Bone Adrenal glands Peritoneum |
5 (20) 5 (20) 1 (4) 1 (4) |
|
Smoking status |
|
|
Ex/never smoker |
14 (74) |
|
Smoker Unknown |
3 (16) 2 (11) |
|
TKI therapy |
|
|
Gefitinib |
17 (89) |
|
Erlotinib |
1 (5) |
|
Afatinib |
1 (5) |
|
Gefitinib |
17 (89) |
Table 1. Patient characteristics.
TKI: Tyrosine Kinase inhibitor.
Quality of life
19 patients (100%) completed FACT-L questionnaires at baseline, 17 (89%) after two weeks, and 15 (79%) after four and eight weeks. Overall, the majority of patients reported stable or improved quality of life. Lung cancer symptoms and TOI (combined score of PWB, FWB and LCS) improved significantly by week 4 and remained stable until the end of the study. Emotional Well-Being improved significantly at the end of the study (Table 2).
|
Subscales |
|
Mean difference from the baseline at week 4 (SD) |
P value (baseline /week 4) |
Mean difference from the baseline at week 8 (SD) |
P value (baseline /week 8) |
|
Baseline Mean (SD1) |
N=16 |
N=16 |
|||
|
N=19 |
|
|
|||
|
|
|
|
|||
|
Physical well-being |
21.09 (3.95) |
0.78 (4.2) |
0.495 |
0.50 (5.4) |
0.735 |
|
Social well- being |
23.7 (4.11) |
-2.25 (4.5) |
0.075 |
-0.30 (5.9) |
0.848 |
|
Emotional well-being* |
17.86 (4.96) |
0.14(3.6) |
0.888 |
3.22 (5.8) |
0.05 |
|
Functional well-being |
17.93 (5.20) |
1.80 (3.7) |
0.082 |
0.73 (3.8) |
0.47 |
|
Lung cancer subscale* |
17.22 (5.16) |
5.26 (9.4) |
0.006 |
3.24 (7.1) |
0.097 |
|
Trial outcome index * |
57.14 (8.91) |
5.1 (12.6) |
0.011 |
4.50 (12.0) |
0.185 |
|
Total |
98.00 (12.86) |
5.15 (6.3) |
0.15 |
3.27 (14.9) |
0.427 |
Table 2: Changes in Functional Assessment of Cancer Therapy-Lung scores.
1-standard deviation
Data are shown as mean (standard deviation). *P< 0.05, vs baseline.
At week 4, none of the 15 patients, who completed FACT-L, had reported worsening, and 12/15 (80%) reported clinically meaningful improvement (≥ 2 points) in lung cancer symptoms (LCS). 8/12 patients reported continues improvement on LCS at week 8. Only one patient reported worsening of TOI index (combined score of PWB, FWB and LCS) at week 4, and 7/15 reported clinically meaningful improvement (≥ 6 points), which continued to improve further in 5/7 patients at week 8 (Figure 3).
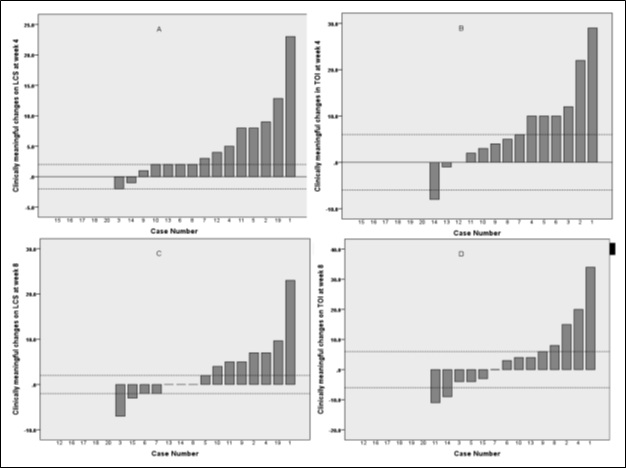 Figure 3. Clinically meaningful changes on Lung Cancer Score (LCS): A - at 4 weeks, C - at 8 weeks; clinically meaningful changes on Trial Outcome Index (TOI) B - at 4 weeks, D - at 8 weeks
Figure 3. Clinically meaningful changes on Lung Cancer Score (LCS): A - at 4 weeks, C - at 8 weeks; clinically meaningful changes on Trial Outcome Index (TOI) B - at 4 weeks, D - at 8 weeks
Safety
Safety was assessed based on reported adverse reactions. Curcumin in combination with TKIs was generally well tolerated. A total of 47 symptoms and/or adverse reactions were reported by study participants in their diary or during scheduled visits and those occurring in 9 patients (Table 3). Diarrhea and skin rash were the most common and were almost exclusively grade 1 or 2. 33/47 (72%) AEs consisted of pre-existing symptoms, presented before starting curcumin; 13 (28%) first occurred during or shortly following the 60-day course. Only one of the 13 reporting new AEs was grade 3 leading to emergency measures and hospitalization: a myocardial infarction in a 65-year-old patient with a 40 pack-year smoking history known for dyslipidemia and peripheral artery disease. No link was found between the use of curcumin and the event. Following hospitalization, the patient continued their 60-day course to completion with no further incidents.
The product was also held due to adverse events on two other occasions: it was stopped indefinitely in one patient due to a persistent grade 1 rash and held for one month in another experiencing significant nausea. Patient’s anti-emetic medication was adjusted during that time, and nausea did not recur after restarting curcumin. These two events were deemed likely related to EGFR-TKIs rather than curcumin. All other adverse events either improved or resolved throughout the treatment course.
|
Type |
Grades |
Present at baseline |
Occurred de novo during the study |
|
|
1-2 |
3-4 |
|||
|
Diarrhea |
5 |
|
4 |
1 |
|
Skin rash |
5 |
|
3 |
2 |
|
Appetite loss |
3 |
|
1 |
2 |
|
Dry skin |
3 |
|
3 |
|
|
Dyspnea |
3 |
|
3 |
|
|
Nausea |
3 |
|
2 |
1 |
|
Chest pain |
2 |
|
2 |
|
|
Cough |
2 |
|
2 |
|
|
Dry eyes |
2 |
|
2 |
|
|
Fatigue |
2 |
|
2 |
|
|
Pruritus |
2 |
|
2 |
|
|
Back pain |
1 |
|
|
1 |
|
Constipation |
1 |
|
1 |
|
|
Dry cough |
1 |
|
|
1 |
|
Dry mouth |
1 |
|
1 |
|
|
Eye infection |
1 |
|
|
1 |
|
Gastroesophageal reflux |
1 |
|
|
1 |
|
Insomnia |
1 |
|
|
1 |
|
Myocardial infarction |
|
1 |
|
1 |
|
Nose bleed |
1 |
|
|
1 |
|
Palpitations |
1 |
|
1 |
|
|
Sore throat |
1 |
|
1 |
|
|
Stomatitis |
1 |
|
1 |
|
|
Vulvitis |
1 |
|
|
1 |
|
Weakness |
1 |
|
1 |
|
|
Weight loss |
1 |
|
1 |
|
|
Total |
46 |
1 |
33 |
14 |
Table 3: Reported adverse events.
The CRP level declined significantly (p<0.05) during the curcumin therapy combined with TKIs from 15.5 g/L at baseline to 7.38 at week 2 and remained decreased during entire treatment course (Figure 4). Level of CRP increased shortly after discontinuation of curcumin (p=0.104). Curcumin had no significant effect on serum aspartate aminotransferase and alanine aminotransferase, alkaline phosphatase, or albumin (Table 4).
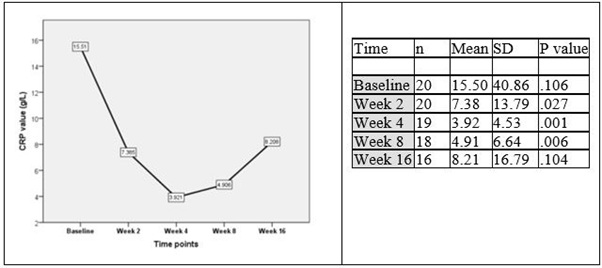 Figure 4: CRP changes during the curcumin treatment.
Figure 4: CRP changes during the curcumin treatment.
|
|
Units |
Baseline Mean(SD) |
Week 4 Mean(SD) |
Mean difference |
P value |
|
Hemoglobin |
g/L |
132.87 (8.39) |
130.20 (9.59) |
-2.66 |
0.018 |
|
White blood cell count |
10^9/L |
6.95 (2.05) |
6.37 (2.15) |
0.58 |
0.536 |
|
C-reactive protein |
g/L |
4.63 (6.75) |
4.49 (4.816) |
-0.14 |
0.906 |
|
Albumin |
mg/L |
40.28 (4.54) |
40.00 (2.32) |
-0.28 |
0.661 |
|
Alkaline phosphatase |
U/L |
134.44 (167.52) |
87.78 (30.41) |
-46.66 |
0.193 |
|
Alanine aminotransferase (ALT) |
U/L |
77.44 (111.53) |
57.28 (94.16) |
-20.16 |
0.534 |
|
Aspartate aminotransferase (AST) |
U/L |
48.16 (48.82) |
37.25 (47.64) |
-10.91 |
0.712 |
Table 4: Hematologic characteristics.
Discussion
This is, to our knowledge the first clinical study of curcumin in combination with EGFR-TKIs for advanced/metastatic EGFR-mutant NSCLC. There is growing interest from patients with cancer to take complementary alternative medicines alongside their standard chemotherapy regimens. As a result, there is a significant interest in developing adjunctive therapies to augment currently available treatment protocols, which may reduce the burden of symptoms associated with standard anti-cancer therapies as well as potentially synergistically improving the efficacy of these regimens. However, most studies based on vitro and animal work that cannot be readily extrapolated to cancer patients [9,21,33,42]. Lack of solid pre-clinical and clinical evidence for their benefits and, more importantly, lack of harm and negative pharmacological interactions urgently required Phase I and II studies to determine safety of their use along with standard chemotherapy [43].
The results presented here demonstrate that interest in curcumin among lung cancer patients is high, as well as compliance and regimen completion rates. Use of curcumin alongside with EGFR-TKIs in EGFR mutant patients were safe; most of reported adverse events were present prior to start of the trial, and the majority of those which occurred following curcumin administration were likely due to TKI use, as consistent with the existing literature [4,44-47]. There were very few adverse events related to curcumin, all of which were mild, and no biochemical evidence of toxicity was observed in the study. Our findings are in line with other Phase I study findings which have reported curcumin to be safe and indicated no dose-limiting toxicity when taken by mouth at doses up to 12,000mg/day [21,28].
Curcumin has also been shown to suppress inflammation in various clinical trials, as revealed by reduction in circulating CRP levels and swelling, as well as histological analysis [48-54]. There exist a number of biological pathways reported in the literature involved in curcumin’s anti-inflammatory properties, namely blockage of NF-κB activation increased by several different inflammatory stimuli, and decreased oxidative stress [42,55]. In this study we demonstrated that CRP level significantly declined after 2 weeks of curcumin treatment and continued to decrease while on treatment. The rise in CRP that observed shortly after stopping curcumin could potentially represent a rebound effect, whose mechanism extends beyond the scope of our study.
Previous randomized controlled trials have demonstrated improvement in quality of life with curcumin supplementation in patients with solid tumors [31]. We did observe significant improvement in Quality of Life, especially regarding lung cancer symptoms as well as physical and functional well-being (as assessed by the Lung Cancer Subscale and Trial Outcome Index of the FACT-L questionnaire). It is difficult, however, to determine the extent to which these changes are due to the beneficial effects of curcumin use, rather than a positive response to EGFR-TKI therapy alone.
Limitations
As any pilot studies, our study is limited because it is not extensive enough to provide conclusive results and was not designed to develop recommendations. The study was not powered to assess treatment effect of curcumin. Thus, given the nature and short duration of this trial, it is difficult to accurately assess the positive effects of curcumin alone and the specific etiology of all reported side effects in the presence of such confounding factors both in the treatment and progression of the disease. Furthermore, our study only examined feasibility of launching large-scale randomized trials in lung cancer patients. The feasibility results do not necessarily generalize beyond the inclusion and exclusion criteria of the pilot. However, pilot studies are a necessary first step in exploring a novel applications of curcumin.
Conclusion
Our small study is the only prospective clinical study of curcumin in advanced/metastatic lung cancer patients which has included safety and toxicity monitoring. As such, it provides preliminary evidence that curcumin alongside EGFR-TKIs is acceptable and safe to use in lung cancer patients. We have learned from this pilot study that a future randomized larger-scale clinical trials using this formulation of curcumin is feasible and safe. Future randomized studies will seek to assess the potential beneficial effects of curcumin with regards to survival and response to TKIs in EGFR mutant advanced lung cancer over more extended periods of time.
Declarations
Sponsor: The study was sponsored by the Research Institute.
Funding: The study received private funding.
Conflicts of interest: The authors declare that they have no conflict of interest.
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Committee and authorized by Health Authorities.
Consent to participate: Written informed consent was obtained from each subject prior to their participation in the study, after adequate explanation of the aims, methods, anticipated benefits, and potential hazards.
Availability of data and material: All non-confidential original study data can be made available upon request.
Author’s contribution: All authors advised on the study protocol and planned statistical analyses. All authors had a role in writing and critical revision of the report.
References
- Society AC (2021) Key Statistics for Lung Cancer. American Cancer Society, USA.
- Walters S, Maringe C, Coleman MP, Peake MD, Butler J, et al. (2013) Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 68: 551-564.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, et al. (2018) Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378: 2078-2092.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380-2388.
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, et al. (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29: 192-237.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246.
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92-98.
- Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, et al. (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26: 2442-2449.
- Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23: 363-398.
- Hewlings SJ, Kalman DS (2017) Curcumin: A Review of Its Effects on Human Health. Foods 6: 92.
- Kotha RR, Luthria DL (2019) Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 24: 2930.
- Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, et al. (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 285: 127-133.
- Shishodia S, Potdar P, Gairola CG, Aggarwal BB (2003) Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 24: 1269-1279.
- Fu S, Kurzrock R (2010) Development of curcumin as an epigenetic agent. Cancer 116: 4670-4676.
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, et al. (2007) Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 67: 3853-3861.
- Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, et al. (2007) Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest 25: 411-418.
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, et al. (2009) Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462: 104-107.
- Wong KK, Jacks T, Dranoff G (2010) Dranoff, NF-kappaB fans the flames of lung carcinogenesis. Cancer Prev Res (Phila) 3: 403-405.
- Stathopoulos GT, Sherrill TP, Han W, Sadikot RT, Yull FE, et al. (2008) Host nuclear factor-kappaB activation potentiates lung cancer metastasis. Mol Cancer Res 6: 364-371.
- Chadalapaka G, Jutooru I, Burghardt R, Safe S (2010) Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res 8: 739-750.
- Chen A, Xu J, Johnson AC (2006) Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 25: 278-287.
- Ye MX, Li Y, Zhang J (2012) Curcumin: updated molecular mechanisms and intervention targets in human lung cancer. Int J Mol Sci 13: 3959-3978.
- Seol DW, Chen Q, Zarnegar R (2000) Transcriptional activation of the hepatocyte growth factor receptor (c-met) gene by its ligand (hepatocyte growth factor) is mediated through AP-1. Oncogene 19: 1132-1137.
- Sakurai R, Li Y, Torday JS, Rehan VK (2011) Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-beta signaling. Am J Physiol Lung Cell Mol Physiol 301: 721-730.
- Zhou H, Beevers CS, Huang S (2011) The targets of curcumin. Curr Drug Targets 12: 332-347.
- Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY, et al. (2011) Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS One 6: 23756.
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, et al. (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21: 2895-900.
- Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, et al. (2006) Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6: 10.
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847-6854.
- Bashang H, Tamma S (2020) The use of curcumin as an effective adjuvant to cancer therapy: A short review. Biotechnol Appl Biochem 67: 171-179.
- Farhood B, Mortezaee K, Goradel NH, Khanlarkhani N, Salehi E, et al. (2019) Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J Cell Physiol 234: 5728-5740.
- Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, et al. (2019) Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J Nutr 149: 1133-1139.
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Mendía LES, et al. (2016) Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother 82: 578-582.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4: 807-818.
- Bhuket PRNB, Magboub AE, Haworth IS, Rojsitthisak P (2017) Enhancement of Curcumin Bioavailability Via the Prodrug Approach: Challenges and Prospects. Eur J Drug Metab Pharmacokinet 42: 341-353.
- Wu X, Xu J, Huang X, Wen C (2011) Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev Ind Pharm 37: 15-23.
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, et al. (2010) Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 58: 2095-2099.
- Gupta T, Singh J, Kaur S, Sandhu S, Singh G, et al. (2020) Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front Bioeng Biotechnol 8: 879.
- Browne RH (1995) On the use of a pilot sample for sample size determination. Stat Med 14: 1933-1940.
- Lancaster GA, Dodd S, Williamson PR (2004) Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 10: 307-312.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, et al. (1995) Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12: 199-220.
- Ashrafizadeh M, Najafi M, Makvandi P, Zarrabi A, Farkhondeh T, et al. (2020) Versatile role of curcumin and its derivatives in lung cancer therapy. J Cell Physiol 235: 9241-9268.
- Sagar SM, Yance D, Wong RK (2006) Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr Oncol 13: 99-107.
- Cersosimo RJ (2006) Gefitinib: an adverse effects profile. Expert Opin Drug Saf 5: 469-479.
- Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, et al. (2012) Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 13: 300-308.
- Reck M, Zandwijk N, Gridelli C, Baliko Z, Rischin D, et al. (2010) Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol 5: 1616-1622.
- Sim EH, Yang IA, Baker RW, Bowman RV, Fong KM (2018) Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev 1: CD006847.
- Deodhar SD, Sethi R, Srimal RC (1980) Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 71: 632-634.
- Holt PR, Katz S, Kirshoff R (2005) Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 50: 2191-2193.
- Martinez N, Herrera M, Frías L, Provencio M, Carrión RP, et al. (2019) A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: results of a pilot study. Clin Transl Oncol 21: 489-498.
- Moghaddam SJ, Barta P, Mirabolfathinejad SG, Aouchiche ZA, Garza NT, et al. (2009) Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis 30: 1949-1956.
- Yuan J, Liu R, Ma Y, Zhang Z, Xie Z (2018) Curcumin Attenuates Airway Inflammation and Airway Remolding by Inhibiting NF-kappaB Signaling and COX-2 in Cigarette Smoke-Induced COPD Mice. Inflammation 41: 1804-1814.
- Gagnon B, Agulnik JS, Gioulbasanis I, Kasymjanova G, Morris D, et al. (2013) Montreal prognostic score: estimating survival of patients with non-small cell lung cancer using clinical biomarkers. Br J Cancer 109: 2066-2071.
- Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, et al. (2010) The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol 17: 52-58.
- Olivera A, Moore TW, Hu F, Brown AP, Sun A, et al. (2012) Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol 12: 368-377.
Citation: Esfahani K, Kasymjanova G, Awan A, Pepe C, Agulnik JS, et al. (2021) Use of Curcumin with Tyrosine Kinase Inhibitors in EGFR-mutant Non-Small Cell Lung Cancer. A Phase I prospective Cohort Trial. J Altern Complement Integr Med 7: 201.
Copyright: © 2021 Kashayar Esfahani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

