
Vaccine Hesitancy and the Question of Data
*Corresponding Author(s):
Eric LeskowitzSpaulding Rehabilitation Hospital, Harvard Medical School, United States
Tel:+1 4136258398,
Email:Rick.leskowitz@gmail.com
Abstract
Public health messaging about Covid vaccines has been confusing to the layperson, and this uncertainty has contributed to widespread vaccine hesitancy in America. Specific areas are outlined, based on several widely reported studies from top-tier medical journals, where existing information could be presented more clearly, or where new data should be developed to answer widespread concerns about vaccine safety and efficacy. Particular issues arise regarding contradictory data about fatal and non-fatal vaccine side-effects, concerns about the quality of vaccine safety research, uncertainty about the preventive impact of vaccines, and distortions regarding how Covid “cases” and “deaths” are reported. These public health measures deserve closer attention from the appropriate agencies overseeing this work.
Overview
Despite assurances by multiple federal health agencies that the Covid-19 vaccines are safe and effective, resistance to these vaccines (“vaccine hesitancy”) is on the rise. Those avoiding vaccination cite concerns about vaccine safety and efficacy, point to disregard of natural immunity post-infection and important lifestyle factors that enhance innate immunity, note inconsistent and contradictory messaging from public health authorities, and perceive restrictions of personal choice by the institution of broad vaccine mandates. While such political factors are beyond the scope of this paper, the safety, efficacy, and messaging concerns cited by vaccine resisters raise important questions about how the quality of scientific evidence is assessed, cited, and disseminated.
Often vaccine hesitancy is justified by arguments disseminated by those espousing unconventional points of view, including Dr. Joseph Mercola, the GreenMedInfo website, Children’s Health Defense, and the so-called “Disinformation Dozen”. Of note, however, a growing number of papers recently published in top-tier medical journals, including several discussed here, support key aspects of this growing counter-narrative. We believe vaccine hesitancy will continue to grow unless compelling evidence addressing these concerns is presented in a straightforward way readily understood by the lay public.
These papers raise six major issues:
- Vaccine safety - What is the true incidence of serious but nonfatal side effects?
- Vaccine safety - What is the true incidence of post-vaccine death?
- Quality of clinical trials - Were adequate precautions taken to ensure appropriate vaccine safety testing was conducted?
- Vaccination efficacy- To what extent do vaccines prevent the spread of Covid?
- Covid case reporting - Does a positive Covid test constitute a true clinical “case”?
- Covid death reports - Is dying “with Covid” the same as dying “from Covid”?
Each of these complex issues merit a systematic literature review to address in depth. In this essay, however, we focus on several recent papers in top-tier journals—the Journal of the American Medical Association (JAMA), The Lancet, the British Medical Journal (BMJ), and the Morbidity and Mortality Weekly Report (MMWR)—whose prominent coverage in the mainstream non-medical media led us to select them for analysis, in order to highlight issues that need to be directly and fully addressed in order to overcome vaccine hesitancy in the general public.
Vaccine Safety: Adverse Events
Two recent epidemiologic studies of the Covid-19 vaccine [1,2] conclude that the risk of vaccination-induced side effects (non-fatal and fatal) are minimal. Such information, if independently validated, and replicated and disseminated widely, could help to stem vaccine hesitancy.
However, methodological issues in these and related recent reports call into question the validity of their conclusions, with two such findings being especially significant. Firstly, an October 2021 article in JAMA¹, “… found no significant associations between vaccination with mRNA COVID-19 vaccines and selected serious health outcomes 1 to 21 days after vaccination.”, and secondly, a November 2021 report in MMWR2 concluded, “There is no increased risk of mortality among Covid-19 vaccine recipients”. We take issue with these conclusions for the following reasons:
Non-Fatal Adverse Events
The Vaccine Adverse Events Reporting System (VAERS) is a national voluntary self-report database operating under the combined aegis of the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA). VAERS was the data source analyzed by Klein et al in JAMA [1]. Data downloaded from VAERS requires careful analysis and should be interpreted with caution because important logistical aspects of VAERS prevent full and accurate data collection: the process to complete VAERS submission forms is long and complex, the diagnoses are unconfirmed by professionals, care providers have been reported to actively discourage their own and their patients’ completion of these forms, and the populace is generally uninformed about existence of this resource [3]. These issues assure cases reported in VAERS are not representative of the general population and should not serve as the basis for generalization.
VAERS reports are further complicated by the late addition of important side-effects like myocarditis to the list of diagnostic options presented to providers and patients to choose from, and by the omission of death as a significant adverse event in the authors’ analysis. These factors contribute to source, reporting, and surveillance biases, leading to under-estimation of event incidence to a significant degree, by one estimate, twenty-fold [4]. This potential for significant under-reporting of side-effects to VAERS vitiates the conclusions in the JAMA report.
Fatal Adverse Events
A Kaiser Permanente research team (that also included the primary author of the JAMA study) followed up with a report in MMWR on post-vaccination mortality rates [2]. In contrast to the VAERS data system, this study utilized the Vaccine Safety Database (VSD), compiled from Kaiser Permanente’s computerized medical record database from 7 clinics in California. The researchers found no increase in aggregate deaths in the first 1-2 weeks post-vaccine, compared to the aggregate totals in weeks 3-4. Of note, raw data from this study are not available for independent analysis. Furthermore, the overly complex statistical analyses used relatively unfamiliar processes as “standard mortality rates compared to rate ratio tests between vaccinated and unvaccinated groups via Poisson modeling of aRR”.
Consider how the data are presented in this paper (Figure 1); only a biostatistician could interpret this number-dense table (to be fair, the article is directed to epidemiologists). However, a wider audience of typical patients and concerned citizens cannot directly benefit from the minutiae of information contained here, at least not in its current form.
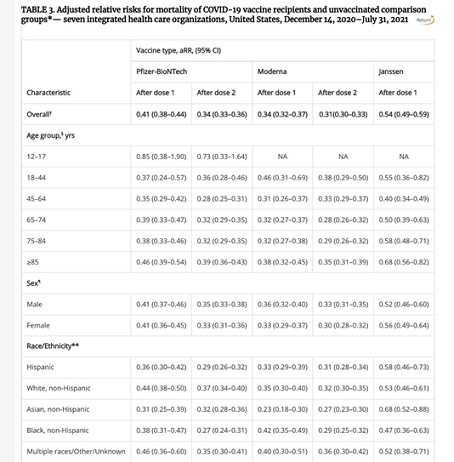 Figure 1: Summary of MMWR data analysis.
Figure 1: Summary of MMWR data analysis.
By contrast, other investigators using the VAERS database have reached a completely different conclusion about death as an adverse event and presented their results in a much more accessible manner. For example, one independent analysis demonstrated an apparent surge of deaths reported in the days immediately following vaccination, and then declining rapidly over the first two weeks post-vaccination [5].
Two points can be made. First, the data are given a much more compelling and understandable presentation in this graph than in the earlier table. More importantly, the reason for this discrepancy in reported death rates between the two analyses is not clear but is obviously a crucial point to resolve. A re-analysis of the MMWR data using the simple graphic presentation of figure 2 charting daily deaths vs. days post-vaccine, starting from the day of the first injection would be an informative next step, and could more readily demonstrate whether death should be further investigated as a notable vaccine side-effect. Unless and until this widely circulated independent analysis is invalidated by other analyses, vaccination acceptance will not increase.
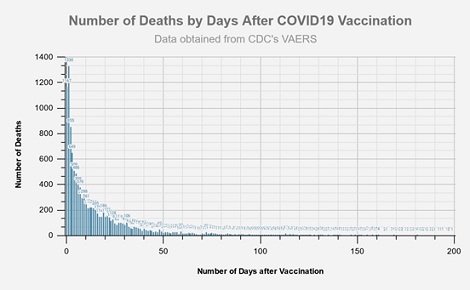 Figure 2: Independent analysis of VAERS data; number of days after vaccination.
Figure 2: Independent analysis of VAERS data; number of days after vaccination.
Quality of Safety Research - Vaccine Testing
A recent BMJ paper “Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight” [6]. raised a whistleblower’s concerns about the field testing of Pfizer’s vaccine research protocols. Furthermore, the usual 5-year study window required by the FDA for establishing vaccine safety has been bypassed by a federal Emergency Use Authorization (EUA) that allowed for only six months of testing and follow-up. Under the EUA, vaccine manufacturers are not constrained by liability fears as they remain indemnified against such legal actions by an earlier Congressional waiver. Policies of expedited regulatory approval make it less likely that rigorous research protocols will be followed, further undermining public confidence in the avowed safety of vaccines [7,8].
Benefits of Vaccination
A recent study in NEJM shows no difference in all-cause death rates between nearly 15 000 vaccinated subjects and 15 000 unvaccinated placebo controls in the Pfizer pre-release trial [9], suggesting the vaccine does not significantly prevent death from Covid-19 infection and, at best, decreases the severity of any subsequent illness during the period of the trial. Similarly, a recent report in The Lancet [10] showed that Covid prevalence rates (measured by RT-PCR status) were as high in a vaccinated population as in a non-vaccinated comparison cohort. In other words, Covid spread was not influenced by the vaccination status of the population in question. By March of 2021, there had been 38 deaths among Pfizer study participants: 17 were in the placebo group and 21 were in the vaccine arm [11]. At that point, however, Pfizer vaccinated the placebo arm study subjects, thereby eliminating the control group and making it impossible to detect further adverse event differences that might have arisen if the study had continued beyond this uncharacteristically brief 6-month window for assessing a novel vaccine.
Claims of high vaccine efficacy have been called into question [12]. Data from Scotland show that since Omicron became dominant in late 2021, COVID case rates and hospital admission rates are lower among the unvaccinated than among the single-, double- and even triple-jabbed [13]. Thus, if vaccine side-effect totals for morbidity and mortality are underestimated, and if vaccine efficacy is over-estimated, then the current strategy of mass mandated vaccination as the primary public health response to Covid must be questioned. In this vein, the evidence standards in medicine and public health remain the randomized clinical trial, which controls for myriad differences in social, political, behavioral, and medical histories of vaccine accepters and refusers. Many evidence-based medicine experts will not acknowledge the causal relationship between increased vaccination and lowered hospitalizations exists until properly conducted and longer-duration trials are completed.
Covid Case Reporting
The most widely used laboratory indicator of Covid’s prevalence has been the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test. Originally developed as a laboratory research tool rather than as a clinical diagnostic, the test is designed to detect specific segments of the viral RNA genome by transcribing the RNA into DNA (reverse transcription - RT) and using a chain reaction to make copies (polymers) of the original sample. However, few studies or agencies rarely mention that the RT-PCR test itself is problematic.
Box 1: PCR - A Quick Overview
The RT-PCR test has several important caveats. Problems arise because the test puts the sample through a series of replications (“amplifications”, or “augmentation cycles”) to make the viral RNA more readily detectable. The number of cycles used (the “cycle threshold” - Ct) determines how often false positives occur. At the high end of this range of replications, even non-viable fragments of the viral genome will have been multiplied enough to be detected and to register as a positive test result [14].
Tests conducted using a Ct of < 25 are generally accepted as accurate, because only a functional viral genome could be detected by these less sensitive but more specific tests; the rate of false positives will be very low. At a Ct value of 30 or above, however, viable virus is recovered from less than 5% of the symptomatic cases, so the rate of false positives is quite high. Also, the ability to culture live virus is shown to decrease by 1/3 for every 1-unit increase in Ct; no study has reported detecting or obtaining cultivable virus from asymptomatic cases [14].
All clinical lab tests, even those with high levels of sensitivity and specificity, will generate more false positives than true positives when the population prevalence of the marker in question is low [15]. But the drawbacks of over-replication are inherent in PCR’s methodology; the result is a bit like making a Xerox copy of a Xerox copy of a Xerox copy the final product will be unrecognizable. Similarly, a very high cycle threshold leads to positive results being triggered by tiny and clinically irrelevant viral fragments, possibly even residual from earlier Covid infection or even from other SARS infections. In summary, a positive PCR is not the definitive sign of active clinical infection, yet Ct threshold values are rarely reported in papers, analyses, and media reports.
Unfortunately, media reports routinely conflate “cases” of Covid with positive RT-PCR test results, regardless of patients’ symptom status and infectivity, and regardless of the Ct value used by the particular testing laboratory. For example, the July 2021 Covid surge in Barnstable County, Massachusetts following multiple outdoor events received much media attention because contact tracing enabled 469 reported potential RT-PCR positive cases to be tracked [16]. What received less attention was that only 5 of these individuals required hospitalization (brief stays not involving ICU-level care) and none died. Yet because this surge occurred at the start of the delta wave, national media generated much anxiety in response to what was, in essence, a large number of positive lab tests with minimal clinical impact.
This disjunct between Covid “cases” and Covid deaths is best illustrated with data generated by the omicron surge, where exponential increases in case rates are being reported in apocalyptic fashion by the media globally. Yet actual all-cause mortality rates world-wide are not keeping pace with this supposed surge, as this graph using Worldometer.com data from 1/25/22 shows in figure 3:
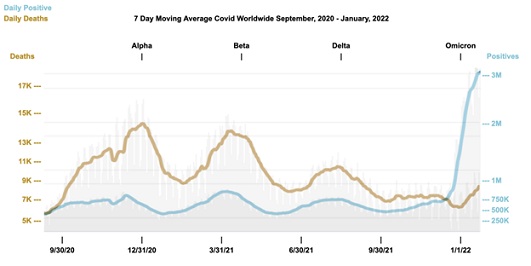 Figure 3: Worldwide comparison of daily positives versus daily deaths (worldometer.com).
Figure 3: Worldwide comparison of daily positives versus daily deaths (worldometer.com).
Of note, starting in 2022, the CDC withdrew Emergency Use Authorization for the RT-PCR testing kits in favor of a new multiplex protocol [17] that will specifically differentiate Covid from flu. Also, during the omicron wave, the popular press began to focus less on reporting “cases” in favor of looking at clinical outcomes like hospitalization rates or fatalities [18]. For example, the state of Massachusetts now distinguishes hospitalized patients who are being treated for Covid symptoms from those who have incidentally tested positive while being treated for unrelated illnesses [19], leading to a 50% decline in the number of reported Covid cases being reported.. In summary, only studies based on tests with specifically noted low thresholds (less than or equal to 25 cycles) and low false positive rates should be considered useful for public health planning, and the threshold levels used in any published report should be clearly specified.
Covid Death Reports
A July 2021 study [20] shows infection fatality rates (i.e., lethality) from Covid may be much lower than previously reported, especially in those under 60 years of age. Conclusions about Covid lethality also are affected by the way how cause of death is determined—“dying with” versus “dying from” Covid. For example, a December 2021 database update by the CDC shows only 6% of Covid-related fatalities that year were diagnosed with Covid alone; that is, they had no medical co-morbidities, and unequivocally “died from” Covid [21]. The other 94% of these deaths were predominantly among older Americans with multiple medical comorbidities; they “died with” Covid. Similarly, UK Covid deaths decreased by 40% when a similar distinction was instituted [22].
Reports circulating in local media and through the “Disinformation Dozen” suggest an unexpectedly high (40%) increase in death from all causes in the under-65 population, as opposed to the seniors who are typically hardest hit. The total is based on actuarial figures from an Indiana insurance company [23] and is being used [24] to promote the notion the Covid vaccines are the cause of these deaths. This is another widely-circulated narrative that should be directly addressed by public health authorities.
Further concerns about the reliability of Pfizer’s clinical trial data were raised when, in response to a Freedom of Information Act filed by a medical transparency group, the FDA requested an unusually long window to respond to the request for information, stating it would take 55 years to process and release the extremely large tranche of Pfizer’s Covid vaccine data [25]. In January 2022, however, a federal court overturned this request and accelerated the release timeline one hundred-fold such that all 300,000 pages would be released by August 2022 [26]. Prompt release of these data may help alleviate concerns about Pfizer’s vaccine’s safety and the integrity of its pre-EUA and approval release testing, as independent researchers can readily analyze the released data.
Summary
Scientific transparency should be the central strategy for addressing vaccine hesitancy. As one researcher stated, there is an “urgent need for the medical and scientific community to anticipate and counter the emergence of falsehoods” [27]. Data recently published in several respected peer reviewed journals call into question the exact relationship between Covid vaccination and the disease itself. Several important research issues merit prompt consideration:
- Data, such as the VSD and Kaiser Permanente, used to decide and promote public health and medical policy should be made available to independent researchers in order to verify and validate findings.
- Analysis of data from the MMWR report can be presented in a more straightforward format, to ensure wider access and acceptance, perhaps using single-day totals rather than 2-week aggregates.
- The VAERS database should be analyzed for death-as-adverse-event, also with single-day rather than 2-week epochs.
- Improved surveillance systems should be less selective, more user-friendly, and more representative than VAERS and VSD, so that harm information can be collected from a more representative sample of vaccine recipients.
- Follow-up of death and other serious events, including medical reports and autopsy records, should be included in VAERS reports or, at the least, analyzed and summarized by health authorities.
- Reported cases should include symptom status (none, mild, moderate, severe) and vaccination status.
- Augmentation cycle thresholds should be specified in all reports about Covid infection and prevalence rates that are based on PCR test results.
Until these questions are adequately and independently addressed through scientifically accurate and widely-disseminated talking point responses, we believe vaccine hesitancy in America and elsewhere will continue to rise.
Acknowledgement
The authors wish to thank Aditi Bhargava MD, Peter Doshi PhD and Andrew Leskowitz for their help in the preparation of this manuscript.
Authors’ Contribution
Dr. Leskowitz did the initial literature search and outline of the paper’s key points. Drs. Simoni and Kaplan have led the review and editing process by clarifying arguments, supplying relevant citations, prioritizing points to be expressed, and rewriting and revising significant portions of the paper. No outside funding or commercial partnerships were involved. No conflicts of interest are reported.
References
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, et al. (2021) Surveillance for Adverse Events After COVID-19 mRNA Vaccination. Journal of the America Medical Association 326: 1390-1399.
- Xu S, Huang R, Sy L, Glenn SC, Ryan DS, et al. (2020) COVID-19 Vaccination and Non–COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021. MMWR Morbidity and Mortality Weekly Report 70: 1520-1524.
- Lazarus R (2010) Electronic Support for Public Health - Vaccine Adverse Event Reporting System (ESP:VAERS) - Final Report. Agency for Healthcare Research and Quality, Rockville, USA.
- Pantazatos S, Seligmann H (2021) VAERS: COVID vaccination and age-stratified all-cause mortality risk.
- VAERS Analysis (2021) VAERS Summary for COVID-19 Vaccines through 8/13/2021, VAERS Analysis, USA.
- Thacker P (2021) Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial. British Medical Journal 375: 2635.
- Mahase E (2020) Covid-19: Vaccine trials need more transparency to enable scrutiny and earn public trust, say experts. BMJ 371: 4042.
- Prugger C, Spelsberg A, Keil U, Erviti J, Doshi P (2021) Evaluating covid-19 vaccine efficacy and safety in the post-authorisation phase. BMJ 375: 067570.
- Sahly HEL, Baden L, Essink B, Lewis SD, Martin JM, et al. (2021) Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. New England Journal of Medicine 385: 1774-1785.
- Kampf G (2021) The epidemiological relevance of the COVID-19-vaccinated population is increasing. The Lancet Regional Health – Europe 11: 100272.
- US Food and Drug Administration. Summary Basis for Regulatory Action. US Food and Drug Administration, USA.
- Doshi P SARS-CoV-2 Variants of Concern in the United States Inaccurate Statement. JAMA Network.
- McArdle H (2022) Covid Scotland: Case rates lowers in unvaccinated as double-jabbed elderly drive rise in hospital admissions. The Herald (Glasgow), USA.
- Jefferson T, Spencer E, Brassey J, Heneghan C (2021) Viral Cultures for Coronavirus Disease 2019 Infectivity Assessment: A Systematic Review. Clin Infect Dis 73: 3884-3899.
- Woloshin S, Patel N, Kesselheim AS (2020) False negative tests for SARS-CoV-2 infection challenges and implications. New England Journal of Medicine 383: 38.
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, et al. (2021) Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR 70: 1059-1062.
- Centers for Disease Control and Prevention (2021) 07/21/21: Lab Alert: Changes to CDC RT-PCR for SARS-CoV-2 Testing. Centers for Disease Control and Prevention, USA.
- Berger E (2022) US experts question whether counting Covid cases is the right approach. The Guardian, USA.
- Fatima S (2022) Here’s why the state is changing the way it reports Covid hospitalization data. Boston Globe, USA.
- Axfors C, Ioannidis J (2021) Infection fatality rate of COVID-19 in community-dwelling populations with emphasis on the elderly: An overview. MedRxiv.
- Centers for Disease Control and Prevention (2019) Weekly Updates by Select Demographic and Geographic Characteristics. Centers for Disease Control and Prevention, USA.
- Knapton S (2022) High Covid death rates skewed by people who died from other causes. The Telegraph (UK), USA.
- Menge M (2022) Indiana life insurance CEO says deaths are up 40% among people ages 18-64. The Elkhart Truth, USA.
- Children's Health Defense (2022) Diamond mine of data? Insurance companies report 40% increase in premature non-Covid deaths. The Defender: Children’s Health Defense, USA.
- Greene J (2021) We’ll all be dead before government releases full Covid vaccine data. Reuters Commentary - Legal Action, USA.
- Public Health And Medical Professionals For Transparency (2022) United States District Court For The Northern District Of Texas Fort Worth Division. P ublic Health And Medical Professionals For Transparency, USA.
- Grimes DR (2021) Medical disinformation and the unviable nature of COVID-19 conspiracy theories. PLoS ONE 16: 0245900.
Citation: Leskowitz E, Wastila L, Kaplan R (2022) Vaccine Hesitancy and the Question of Data. J Altern Complement Integr Med 8: 263.
Copyright: © 2022 Eric Leskowitz, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

