
What’s Hidden Under the Gastric Intestinal Metaplasia? Diffuse-Type Adenocarcinoma Discovered by Targeted Biopsies: A Case-Report
*Corresponding Author(s):
Edith LahnerDepartment Of Medical-Surgical Sciences And Translational Medicine, Sant’ Andrea Hospital, Sapienza University Of Rome, Via Grottarossa 1035, 00189 Rome, Italy
Tel:00390633775696,
Email:edith.lahner@uniroma1.it
Abstract
Gastric corpus atrophy and intestinal metaplasia are two well established gastric precancerous conditions and scientific evidence shows a confidential relationship between this precancerous condition and intestinal type gastric cancer, according to Lauren classification. Differently, the background histological gastric mucosa characteristics from whom diffuse type gastric cancer may arise, need to be clarified. In this case, report we present a case of an 81-year-old female with corpus atrophic gastritis in whom we arrived at the diagnosis of diffuse type gastric cancer by following specific guidelines related to the characterization and the endoscopic-histological surveillance of gastric precancerous conditions.
Introduction
Gastric cancer still remains a major public health concern. Most of gastric cancerous lesions arise from atrophic gastritis, a well-known gastric precancerous condition characterized by a chronic inflammation process leading to the progressive loss of canonical gastric glands [1].
By the time, the inflammation process may give arise to a progressive replacement of original gastric glands by intestinal metaplasia, as described in the Correa cascade, up to the eventual development of intestinal type gastric cancer, according to Lauren classification [2,3]. The annual incidence of intestinal type gastric cancer has been reported to be 0.1%-0.25% in patients with chronic atrophic gastritis and 0.25% in patients with intestinal metaplasia and may be high as 1.36% person-year for any gastric neoplasia (including dysplasia and neuroendocrine tumors) [4, 5]. Cumulative incidence of intestinal type gastric cancer of 2.4% at 10 years in patients with intestinal metaplasia were reported, and a Swedish study reported a cumulative incidence at 20 years of approximately 2% in patients with atrophic gastritis and 2.5% in patients with intestinal metaplasia [6]. More recently, a cumulative incidence of 1.7% of intestinal type gastric cancer was reported in patients with corpus intestinal metaplasia [7].
Differently, less is known about relationship between gastric precancerous conditions and diffuse type gastric cancer. Histopathological and endoscopic characterization of the gastric mucosa remain a milestone in the evaluation of predictive features and for a better patient-based risk stratification regarding gastric precancerous conditions. Moreover, surveillance of atrophy and intestinal metaplasia may not only improve early intestinal type gastric cancer detection, but also diffuse type gastric cancer. This type of gastric cancer, differently from the intestinal-type, seems to occur at younger age, equally afflicts males and females, and more commonly originates in the proximal stomach [8]. Far less is understood about the pathogenesis of the diffuse type gastric cancer, whose development may have a stronger genetic contribution than intestinal type gastric. However, recently, a growing interest developed for a better characterization of gastric cancer type. A recent meta-analysis by Wang et al. [9], reported that both, atrophy and intestinal metaplasia, were associated with diffuse type gastric cancer (pooled OR 1.9, 95%CI 1.5-2.4 p < 0.001; pooled OR 2.3, 95%CI 1.9-2.9 p < 0.001, respectively) albeit it is not included in the Correa cascade.
Case Presentation
We report the case of an 81-year-old female with corpus atrophic gastritis diagnosed during gastroscopy performed in another hospital for dyspepsia. No macroscopic alterations were reported. Random biopsies collected from antrum and corpus showed gastric atrophy and intestinal metaplasia restricted to the corpus and presence of Helicobacter pylori infection both, in antrum and corpus. The patient underwent single-pill quadruple bismuth-based therapy for 10 days and 6 months later, in June 2021, she was referred to our GI endoscopy unit for gastroscopy checking-out eventual Helicobacter pylori eradication.
We performed gastroscopy according to MAPS II guidelines [10], and performance measures for upper GI endoscopy [11] were applied. Again, no macroscopic alterations were reported (Figure 1). At virtual chromoendoscopy with Blue Light Imaging (Figure 2), we noticed diffuse areas of intestinal metaplasia in the gastric corpus, so target biopsies were collected in addition to those according to updated Sydney system [12]. At histopathological evaluation, gastric atrophy, pseudopyloric and intestinal metaplasia were present in gastric corpus; no alterations were reported in antrum. Helicobacter pylori was not detected in both, antrum and corpus. In one corpus sample, characterized by intestinal metaplasia at BLI, the presence of diffuse type gastric cancer was reported. The patient underwent CT total body scan with intravenous contrast showing a slight greater wall thickness of the small curvature of the gastric corpus without enhancement (Figure 3). No pathological lymph nodes were observed. Finally, the patient underwent total gastrectomy. The histopathological evaluation described the presence of diffuse type gastric cancer of 2 mm of diameter on the small curvature of corpus. The remaining gastric corpus mucosa showed gastric atrophy with pseudopyloric and intestinal metaplasia. Moreover, extensive corpus sampling showed numerous well differentiated type 1 neuroendocrine tumors G1; antrum was spared. The lymph nodes evaluated at immunohistochemistry (for CKAE1/AE3) reported no neoplastic repetition. After 1 year, the patient is alive.
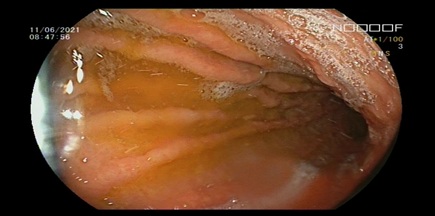 Figure 1: Gastric endoscopic evaluation at white light mode.
Figure 1: Gastric endoscopic evaluation at white light mode.
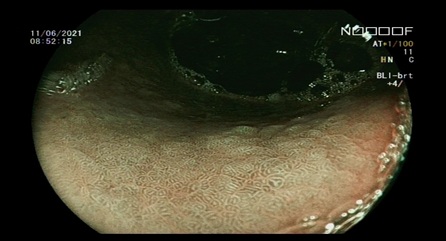 Figure 2: Gastric endoscopic evaluation with virtual chromoendoscopic. The Blue Light Imaging mode shows diffuse presence of gastric intestinal metaplasia.
Figure 2: Gastric endoscopic evaluation with virtual chromoendoscopic. The Blue Light Imaging mode shows diffuse presence of gastric intestinal metaplasia.
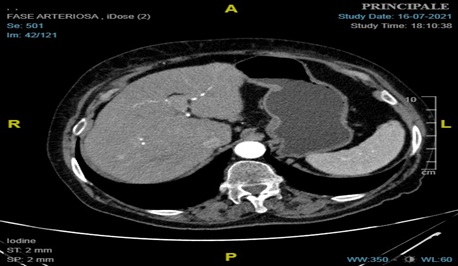 Figure 3: CT axial scan showing a slight greater wall thickness of the small curvature of the gastric corpus without enhancement.
Figure 3: CT axial scan showing a slight greater wall thickness of the small curvature of the gastric corpus without enhancement.
Discussion
Early recognition of gastric neoplastic lesions should be considered one of the major goals of the endoscopic evaluation. Even though early detection and treatment is possible, most cases are diagnosed at a late stage and thus most patients with a diagnosis of gastric cancer die of the disease. Screening and surveillance of people at risk may decrease gastric cancer mortality by allowing early diagnosis and treatment, often by endoscopy instead of more invasive surgery. Corpus atrophic gastritis and intestinal metaplasia are considered to be precancerous conditions because they independently confer a risk for development of gastric cancer and constitute the background in which dysplasia and adenocarcinoma may occur [4-7].
Accomplishing specific recommendations for the management of patients with corpus atrophic gastritis should be prioritized. Gastroscopy may be considered a necessary tool in the diagnostic work-up of atrophic gastritis patients, since during the endoscopic examination gastric biopsies can be taken, which are necessary to establish a diagnosis of corpus atrophic gastritis by histopathological evaluation. For adequate staging of atrophy, gastroscopy should include gastric biopsies according to the biopsy protocol of the updated Sydney system, which recommends two antral samples, one sample from incisura angularis, and two samples from corpus [12]. Furthermore, it should be highlighted the recent endoscopic advance, virtual (or electronic) chromo endoscopy, which, whenever available and after proper training, is a valuable tool, since it allows, among the other uses, to characterize gastric mucosa highlighting eventual gastric areas suspected of intestinal metaplasia or neoplastic lesions, shifting from random biopsies to “targeted biopsies” [10]. It should be underlined that considerable efforts were done to provide specific standard quality statements in order to perform a high-quality upper gastrointestinal evaluation [11]. Observation, characterization, and performing biopsies according to guidelines, may be the standard of care for Endoscopist [13].
This report suggests that following guidelines for the management of gastric neoplastic conditions, applying the performance measures for quality gastroscopy and virtual chromo endoscopy permit correct diagnosis of potentially life-threatening lesions as diffuse type gastric cancer whose diagnosis might have been easily missed without this methodology. The association between gastric precancerous conditions and diffuse type gastric cancer is intriguing and needs further investigation.
Acknowledgments
ED is a PhD student at Sapienza University of Rome.
References
- Lahner E, Zagari RM, Zullo A, Di Sabatino A, Meggio A, et al. (2019) Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver; 51: 1621-1632.
- Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res; 52: 6735-40.
- Lauren P (1965) The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 64: 31-49.
- de Vries AC, van Grieken NCT, Looman CWN, Casparie MK, de Vries E, et al. (2008) Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology; 134: 945-52.
- Lahner E, Esposito G, Pilozzi E, Purchiaroni F, Corleto VD, et al. (2015) Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol; 50: 856-65.
- Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, et al. (2015) Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ; 351: h3867.
- Dilaghi E, Baldaro F, Pilozzi E, Conti L, Palumbo A, et al. (2021) Pseudopyloric Metaplasia Is Not Associated With the Development of Gastric Cancer. Am J Gastroenterol; 116: 1859-67.
- Assumpção PP, Barra WF, Ishak G, Coelho LGV, Coimbra FJF, et al. The diffuse-type gastric cancer epidemiology enigma. BMC Gastroenterol; 20: 223.
- Wang JE, Kim SE, Lee BE, Park S, Hwang JH, et al. (2022) The risk of diffuse-type gastric cancer following diagnosis with gastric precancerous lesions: a systematic review and meta-analysis. Cancer Causes Control CCC; 33: 183-91.
- Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, et al. (2019) Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy; 51: 365-88.
- Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, et al. (2016) Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 48: 843-64.
- Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol; 20: 1161-81.
- Lahner E, Zullo A, Hassan C, Perri F, Dinis-Ribeiro M, et al. (2014) Detection of gastric precancerous conditions in daily clinical practice: a nationwide survey. Helicobacter; 19: 417-24.
Citation: Emanuele D, Stefano A, Gianluca E, Emanuela P, Bruno A, et al. (2023) What’s Hidden Under the Gastric Intestinal Metaplasia? Diffuse-Type Adenocarcinoma Discovered by Targeted Biopsies: A Case-Report. J Clin Stud Med Case Rep 10: 0159.
Copyright: © 2023 Dilaghi Emanuele, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

