
Why Is Wood-Feeder, Apate Terebrans Attacking Only Terminalia Mantaly In The Midst Of Susceptible Urban Tree Species? Evaluation of Wood Feeding Shift Hypotheses
*Corresponding Author(s):
Adedeji GADepartment Of Forestry And Wildlife Management, University Of Port Harcourt, Nigeria
Email:gabriel.adedeji@uniport.edu.ng
Abstract
The Wood-Feeding Shift Hypotheses (WFSH) posits that Apate terebrans may not shift (H0) or shift (H1) feeding on Terminalia mantaly in the midst of susceptible species. The WFSH was tested by feeding preference, moisture contents, and chemical elements evaluations of 5 popular urban forest species with and without T. mantaly wood samples under laboratory conditions. Spondias mombin was most highly preferred followed by T. mantaly, and Gmelina arborea. Delonix regia and Azadirachta indica were less-preferred and Tectona grandis was not preferred. The mean Moisture Content (MC) of the Wood Samples (WS) ranged from 79.70 ± 2.24% to 115.16 ± 4.32%. The Particle-Induced X-ray Emission (PIXE) analysis revealed common seven-element [Magnesium (Mg), Aluminum (Al), Phosphorus (P), Sulfur (S), Iron (Fe), Potassium (K), Calcium (Ca)] among the WS. Al ~ S and MC ~ K revealed a significant highest relationship for element-wise and MC ~ element relationship, respectively at p<0.01. The results of this study supported the alternative hypothesis (H1) that there is a shift in the feeding preferences of A. terebrans. We inferred that S. mombin and/or G. arborea trees may likely be the next shift hosts of A. terebrans. The highest concentrations of Al, P, S, Fe, and K in S. mombin might be responsible for its highest feeding preference by A. terebrans.
Keywords
Apate terebrans; Chemical elements; Feeding preferences; Moisture contents; Plant hosts
Introduction
Apate terebrans Pallas, 1772 (Coleoptera: Bostrichidae) is a polyphagous wood-feeding insect pest of global importance due to the serious damage it causes to woody trees including agricultural crops. Across the globe, this species is one of the major pests of some of the most economically important agricultural crops; Theobroma cacao, Citrus spp., Anacardium occidentale [1,2], and forest crops; Azadirachta indica, Khaya spp., Tectona grandis [3-5]. A. terebrans is a primary insect pest, whose adults infest mainly wood of healthy trees causing serious mechanical damage and the death of trees [6,7]. The adults live and feed on living wood but the oviposition by females is done on dead wood [3,8].
The survey of literature revealed several hosts of A. terebrans worldwide with a history of three species: Delonix regia [6]; A. indica [9]; Terminalia mantaly [10] in Nigeria. The report of A. terebrans in Nigeria was probably first dated 1867 by Murray [11], though the wood species on which the beetle was found was not documented. The resurgence of A. terebrans since 2015, as a principal and important pest of T. mantaly, is of great environmental concern in Nigeria [12] with quick killing impact due to yearly repeated attacks [13]. T. mantaly has not only being one of the highly hazardous urban trees causing human death in Malaysia [14], but also represents a national threat to human, wood-based industries, cash, and food securities in Nigeria due to its characters and its potential associated entomological problems [13].
While the plant’s feeding hosts reported in Nigeria and elsewhere are well established in Nigeria landscapes, the infestation of only T. mantaly in the midst of susceptible species remains unclear. T. mantaly is native to Madagascar [15], a hotspot origin of Bostrichidae worldwide [16]. Thus, the current resurgence of A. terebrans is thought to be largely due to the trade of importing seedlings or planting stocks of T. mantaly from Madagascar. Nigeria is one of the most importing countries of inputs worldwide and the utilisation of T. mantaly as one of the popular ornamental trees [17-19] has come with a serious entomological problem [12]. The tendency of polyphagous insects to restrict their feeding to a specific host and really act as oligophages in the presence of other feeding hosts has been attributed to the population advantage of such specific host [20]. As unchecked, the increasing building up of A. terebrans population might reach a threshold for shifting host. Understanding why a particular woody tree species become successful host of polyphagous insect pests in the midst of many susceptible species is an important research interest in wood degradation and invasion/chemical ecology [21,22]. The concurrent infestation of multiple trees including T. mantaly in the neighbouring country, the Republic of Benin has been previously documented [2]. While wood is a nutritionally valuable plant material for xylem-feeding insects, its levels of moisture and chemical nutrients are important cues to its preferences [23]. With the current situation, the anticipated population explosion may cause a shift of hosts. Understanding the likely next host(s) is important in environments, where multiples of the environmental trees are on the red list of A. terebrans attacks. The study that seeks to understand the likelihood of the next shift host is crucial for tree health and biosecurity in Nigeria. Hence, it was hypothesized that A. terebrans would not shift (H0) or shift (H1) feeding on T. mantaly in the midst of susceptible species. Moisture content (MC) and chemical elements were then evaluated to further elucidate their roles in feeding preference(s) occurring in the model beetle.
Materials And Methods
Laboratory feeding preference trials
For wood feeding preference, choice-feeding method was adopted. Six popular urban woody tree species (A. indica, D. regia, G. arborea, T. grandis, T. mantaly and S. mombin) were selected. S. mombin was included due to its susceptibility to wood-girdler, Analeptes trifasciata (Figure 1) The wood feeding preference trials were experimented under laboratory conditions (temperature 26 ± 2°C in 75 ± 5% relative humidity) at the Analytical Wood Laboratory Unit of the Department of Forestry and Wildlife Management, UNIPORT for a duration of four-week. Two types of experimental trials were performed:
- With green wood samples.
- With oven-dry wood samples (with and without T. mantaly).
 Figure 1: Analeptes trifasciata girdling S. mombin.
Figure 1: Analeptes trifasciata girdling S. mombin.
For first trial, green wood samples were used. Six freshly selected wood species of the same length (8cm) but variable sizes ranging between 8.96 cm and 10.4 cm in circumference were placed into 75cl glass bottles fitted with ventilated lids. Four adults of A. terebrans collected from T. mantaly were released into the bottle containing green samples of the six selected wood species. This experiment was replicated twice as W1.1 and W1.2. Under the first trial, another experiment was conducted with the exclusion of reference wood, T. mantaly. In this experiment, five wood species were employed but with similar procedures and replication as Wt1.1 and Wt1.2.
For the second trial, oven-dried wood species were used. In this case, the stem bark of the wood samples were removed, oven dried at 1050C for 16 hours, and conditioned at room temperature prior to test. Similarly, four adults of A. terebrans collected from T. mantaly were released into the bottle containing oven dried samples of the six selected wood species. The experiment was repeated twice with inclusion of T. mantaly (W2.1 and W2.2) and with exclusion of T. mantaly (Wt2.1 and Wt2.2).
Moisture Contents (MC) evaluations
Fresh twigs of similar length (6 cm) with circumferences ranging between 7.60 cm and 8.87 cm were harvested, both ends sealed with nylon cello-tape, and taken to laboratory for the measurement of green weight. Six replicates for each of the selected wood species were used for the computation of the mean MC. The samples were oven dried at 1050C to a constant weight. The relative MC and its coefficient of variation (CV) were evaluated by equations (1) and 2, respectively:
 Where;
Where;
MC (%)
GW = green weight (g)
OW = oven dry weight (g)
 Where;
Where;
CV (%)
SD = Standard deviation
X = Mean (%)
PIXE elementals analysis of wood species samples
The Particle-Induced X-ray Emission (PIXE) elementals analysis of wood species samples was carried out as describe by Ofodile et al. [24]. The dried wood samples were ground, mixed with 10% by weight of ultra-pure graphite powder to produce thick pellets of 13mm diameter without a binder. The analysis was performed on a NEC 5SDH 1.7MV Pelletron Accelerator equipped with a radiofrequency charge exchange ion source, 2.5 MeV proton beam analysis (IBA) facility. The end-station consists of an Aluminium chamber of about 150cm diameter and 180 cm height. It has four ports and a window. Port 1 at 165o is for the RBS detector, port 2 at 135o is for PIXE detector, and port 3 at 30o is for the ERDA detector, the window at 00 is for observing the beam position and the size, while port 4 at 270o is for PIGE. The chamber has a sample ladder that can carry 11 (eleven) 13mm diameter samples. The end-station has a turbo pump and a variable beam collimator to regulate beam size, and an isolation value. Apple leaves standard (NIST 1515) was used for determining of the H-value which was subsequently used for analyzing the gallstone samples and to assure the accuracy of the experimental procedure. The measurements were carried out with a beam spot of 4mm in diameter and a low beam current of 3-6nA. The irradiation was about 10 -20 minutes. A Canberra Si (Li) detector model ESLX 30-150, beryllium thickness of 25μm, with Full Width Half Maximum (FWIIM) of 150eV at 5.9keV, with the associated pulse processing electronics, and a Canberra Genie 2000 (3.1) MCA card interfaced to a PC were used for the X-rays data acquisition. With respect to the beam director, the sample’s normal was located at 0o and the Si (Li) detector at 45o. The PIXE set-up was calibrated using some pure element standards and NIST geological standard, NBS278. The computer code GUPIXWIN (4) was used for the analysis of the PIXE data. This provided non-linear lest square fitting of the spectrum, together with subsequent conversion of the fitted X-ray peak intensities into elemental concentrations, utilizing fundamental parameter method for quantitative analysis.
Statistical analyses
Feeding preference of A. terebrans on selected wood species was qualitatively analyzed and visually categorized into superficial feeding, in-depths feeding and no feeding. A one-way analysis of variance (ANOVA) was employed in determining moisture content differences amongst the wood species at 0.05 levels of significance and means separated using Fisher’s Least Significant Difference (LSD). Also, one-way ANOVA was used in evaluating the concentration of essential nutrient elements among the selected species. In assessing the underlying relationship between moisture content and elements, Principal Component Analysis (PCA) was carried out along with Pearson’s correlation with significance considered at 0.05 and 0.01 levels. One-way ANOVA and LSD post hoc test was done using IBM SPSS (v. 4.1.1) while R software version 4.1.1 was utilized for PCA and plot3D package (v. 1.4) for PCA visualization, correlation matrix of elements and moisture content was produced using R software base package.
Results And Discussion
Feeding preference
As shown in Table 1, and Figures 2-4, the wood-feeding results indicated broad preferences, reaffirming A. terebrans as a polyphagous wood-feeder. The unsatisfactory results from using green wood samples informed the second trial experiments employing oven-dry wood specimens. Both at the green and dry states, wood-feeding preferences were found in all the wood species samples except Tectona grandis (Table 1) indicating its non-preference. The wood-feeding preferences were classical: in-depth feeding reflecting high preference (Figures 2-4); superficial feeding reflecting less preference; and no feeding reflecting non-preference (Table 1). A. terebrans showed a high preference for S. mombin, T. mantaly, and G. arborea wood species with in-depths feeding of 2.7cm, 2.6cm, and 1.2cm, respectively (Figures 2-4). Less preference was shown for A. indica and D. regia, and non-preference was shown for T. grandis (Table 1). Test wood species at green state experiments were found with massive colonization of unidentified fungi and then infestation of Drosophila melanogaster. This situation was probably responsible for the only superficial feeding patterns found and presented in Table 1. Also, the death of all the samples of A. terebrans occurred within two weeks of the experimental period of four-week. Wood being one of the most important resources in providing both food and habitat for multigenerational development and reproduction of many wood-feeding insects [25,26], T. grandis was not preferred and was wholly avoided by A. terebrans throughout the experimental trials. Although T. grandis at the nursery/tending field stage has been listed as feeding host plant of this beetle in Benin, Ghana, and Zambia [27], its non-selection for feeding in this study may be explained probably by the age variability. Less preference and death of A. terebrans found within two weeks when green wood samples were used may be attributed to antagonistic impacts of unidentified fungi interaction with the samples. This further confirms the adult beetle as a serious pest avoiding decaying or weak wood samples (trees) but prefers healthy ones. The in-depth feeding preferences exhibited by A. terebrans to S. mombin and G. arborea highlights the hosts’ shifting potential of the beetle for utilising both sound wood under service and healthy woody tree species. The highest concentrations of Al, P, S, Fe and K in S. mombin might be the cue responsible for its highest feeding preference.
|
Experiments |
|
Levels of feeding at green state |
||||||||||||||||||
|
1 |
|
A. indica |
D. regia |
|
G. arborea |
S. mombin |
T. grandis |
T. mantaly |
||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
W1.1 |
|
x |
* |
|
x |
X |
x |
* |
||||||||||||
|
W1.2 |
|
x |
* |
|
x |
X |
x |
* |
||||||||||||
|
Wt1.1 |
|
x |
* |
|
* |
X |
x |
- |
||||||||||||
|
Wt1.2 |
|
x |
* |
|
x |
X |
x |
- |
||||||||||||
|
2 |
Levels of feeding at dry state |
|||||||||||||||||||
|
W2.1 |
|
* |
|
* |
|
x |
|
** |
|
x |
|
* |
||||||||
|
W2.2 |
|
* |
|
* |
|
x |
|
* |
|
x |
|
** |
||||||||
|
Wt2.1 |
|
x |
|
x |
|
* |
|
* |
|
x |
|
- |
||||||||
|
Wt2.2 |
|
x |
|
x |
|
** |
|
* |
|
x |
|
- |
||||||||
Table 1: Feeding preference of A. terebrans on selected susceptible wood species.
Note: *Superficial feeding, ** In-depths feeding, x No feeding, - Not included, W (with T. mantaly) Wt (without T. mantaly)
 Figure 2: In-depth feeding on S. mombin by A. terebrans.
Figure 2: In-depth feeding on S. mombin by A. terebrans.
 Figure 3: In-depth feeding on T. mantaly by A. terebran.
Figure 3: In-depth feeding on T. mantaly by A. terebran.
 Figure 4: In-depth feeding on G. arborea by A. terebrans.
Figure 4: In-depth feeding on G. arborea by A. terebrans.
Moisture Content (MC) and chemical elements
The mean MC of the tested wood species samples ranged from 79.70 ± 2.24% to 115.16 ± 4.32% with significant differences. The highest coefficient of variation for MC was found in Gmelina arborea (Table 2). The total chemical elements varied considerably (Table 3) with seven essential elements (Mg, Al, P, S, Fe, K, Ca) common in all the tested wood species (Table 4). The essential elements constituted the largest quantitative component of the elemental composition of wood species associated with A. terebrans nutrition. The Principal Component (PC) analysis was performed on essential elemental composition and Moisture Content (MC) (n=8) of six (6) selected wood species.
|
Species |
|
Mean MC ± SD (%) |
|
Range (%) |
|
CV (%) |
|
Delonix regia |
|
115.16 ± 4.32a |
|
107.89-119.79 |
|
3.75 |
|
|
|
|
|
|
|
|
|
Terminalia mantaly |
|
111.83 ± 6.74a |
|
103.06-121.52 |
|
6.03 |
|
|
|
|
|
|
|
|
|
Spondias mombin |
|
110.17 ± 5.51a |
|
100.00-114.63 |
|
5.00 |
|
Azadirachta indica |
|
90.01 ± 9.96b |
|
76.09-100.00 |
|
11.07 |
|
Gmelina arborea |
|
80.53 ± 11.30c |
|
70.33-101.22 |
|
14.03 |
|
Tectona grandis |
|
79.70 ± 2.24c |
|
76.25-83.33 |
|
2.81 |
Table 2: Moisture content of selected wood species samples.
|
Elements |
Concentrations (mg/kg) Mean±S.E |
|||||||||
|
A. indica |
|
D. regia |
G. arborea |
T. grandis |
|
T. mantaly |
|
S. mombin |
||
|
|
|
|
|
|
|
|
|
|
||
|
Mg |
1133.60 ± 65.46 |
|
1040.80 ± 55.45 |
710.95 ± 43.59 |
2730.55 ± 56.46 |
|
701.95 ± 23.38 |
|
808.90 ± 127.53 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Al |
164.30 ± 19.07 |
|
98.65 ± 7.38 |
116.20 ± 19.49 |
121.80 ± 7.92 |
|
102.65 ± 18.89 |
|
173.55 ± 27.62 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Si |
270.60 ± 11.73 |
|
153.90 ± 7.03 |
207.55 ± 31.84 |
286.35 ± 20.66 |
|
132.10 ± 4.44 |
|
319.15 ± 57.13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P |
1116.40 ± 66.66 |
|
1455.30 ± 15.93 |
514.35 ± 8.99 |
1062.45 ± 9.43 |
|
494.00 ± 51.86 |
|
1914.85 ± 578.75 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
970.05 ±2 4.01 |
|
519.45 ± 8.22 |
633.60 ± 32.54 |
727.45 ± 43.70 |
|
516.25 ± 11.04 |
|
1097.70 ± 240.41 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
K |
4928.50 ± 58.38 |
|
6850.90 ± 11.95 |
3166.90 ± 109.85 |
4258.90 ± 221.49 |
|
7439.40 ± 38.35 |
|
12183.25 ± 2698.54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ca |
4769.30 ± 223.05 |
|
3874.30 ± 391.60 |
2912.55 ± 122.86 |
13725.60 ± 505.79 |
|
8615.35 ± 1019.93 |
|
7835.75 ± 1907.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe |
186.80 ± 21.74 |
|
196.35 ± 4.82 |
90.00 ± 10.38 |
165.80 ± 21.33 |
|
221.65 ± 9.80 |
|
224.90 ± 54.94 |
|
|
Cr |
32.70 ± 4.53 |
|
30.45 ± 3.21 |
18.65 ± 2.42 |
ND |
|
43.40 ± 2.93 |
|
ND |
|
|
Cl- |
1384.60 ± 82.94 |
|
628.60 ± 7.09 |
ND |
ND |
|
1823.40 ± 50.89 |
|
1619.65 ± 355.03 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mn |
ND |
|
ND |
25.55 ± 1.94 |
ND |
|
ND |
|
ND |
|
|
Zn |
ND |
|
ND |
19.85 ± 3.20 |
ND |
|
ND |
|
ND |
|
Table 3: Total elemental composition in selected wood species samples.
|
Elements |
Concentration (mg/kg) Mean±S.E |
|||||||
|
A. indica |
D. regia |
G. arborea |
T. grandis |
T. mantaly |
|
S. mombin |
||
|
Mg |
1133.60 ± 65.46b |
1040.80 ± 55.45b |
710.95 ± 43.59c |
2730.55 ± 56.46a |
701.95±23.38c |
|
808.90 ± 127.53c |
|
|
|
|
|
|
|
|
|
|
|
|
Al |
164.30 ± 19.07a |
98.65 ± 7.38b |
116.20 ± 19.49ab |
121.80 ± 7.92ab |
102.65±18.89b |
|
173.55 ± 27.62a |
|
|
|
|
|
|
|
|
|
|
|
|
P |
1116.40 ± 66.66bc |
1455.30 ± 15.93ab |
514.35 ± 8.99c |
1062.45 ± 9.43bc |
494.00±51.86c |
|
1914.85 ± 578.75a |
|
|
S |
970.05 ± 24.01ab |
519.45 ± 8.22c |
633.60 ± 32.54c |
727.45 ± 43.70bc |
516.25±11.04c |
|
1097.70 ± 240.41a |
|
|
|
|
|
|
|
|
|
|
|
|
Fe |
186.80 ± 21.74a |
196.35 ± 4.82a |
90.00 ± 10.38b |
165.80 ± 21.33ab |
221.65±9.80a |
|
224.90 ± 54.94a |
|
|
|
|
|
|
|
|
|
|
|
|
K |
4928.50 ± 58.38bc |
6850.90 ± 11.95b |
3166.90 ± 109.85c |
4258.90 ± 221.49bc |
7439.40±38.35b |
|
12183.25 ± 2698.54a |
|
|
|
|
|
|
|
|
|
|
|
|
Ca |
4769.30 ± 223.05c |
3874.30 ± 391.60c |
2912.55 ± 122.86c |
13725.60 ± 505.79a |
8615.35±1019.93b |
|
7835.75 ± 1907.02b |
|
Table 4: Comparison of common essential elements among the selected wood species samples.
Explained variability for the first three PCs cumulated at 90.11% with PC1 explaining 48.33%, PC2 26.31% and PC3 15.47%. Elements with high loading score which are best explained by PC1 include P, S, Fe, and K, PC2 and were well defined by Mg and MC. Ca had similar loading magnitude for PC2 and PC3 while Al was the most effective variable for PC3 (Figure 5). Mg was significantly correlated with Ca (0.74) and MC (-0.52), Al, S, Fe and K all exhibited positive correlation with each or with r>0.4. MC was also observed to be significantly correlated with Fe and K while Fe was also correlated with Ca (Table 5).
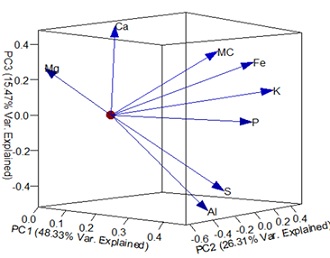 Figure 5: Plot of loadings of components 1, 2 and 3.
Figure 5: Plot of loadings of components 1, 2 and 3.
|
|
|
Mg |
|
Al |
|
P |
|
S |
|
Fe |
|
K |
|
Ca |
|
MC |
|
Mg |
|
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Al |
|
0.03 |
|
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
P |
|
0.14 |
|
0.59** |
|
1.00 |
|
|
|
|
|
|
|
|
|
|
|
S |
|
0.10 |
|
0.82** |
|
0.76** |
|
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe |
|
0.02 |
|
0.44* |
|
0.68** |
|
0.54** |
|
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
K |
|
-0.22 |
|
0.47* |
|
0.80** |
|
0.62** |
|
0.78** |
|
1.00 |
|
|
|
|
|
Ca |
|
0.74** |
|
0.12 |
|
0.27 |
|
0.26 |
|
0.42* |
|
0.27 |
|
1.00 |
|
|
|
MC |
|
-0.52** |
|
-0.07 |
|
0.32 |
|
-0.09 |
|
0.55** |
|
0.64** |
|
-0.15 |
|
1.00 |
Table 5: Correlation matrix for the essential element-wise and MC ~ element.
*p < 0.05; **p < 0.01
The importance of the high MC and availability of chemical elements for A. terebrans nutrition has been demonstrated in this study. The common elements found in this study showed the greater potential of nutrient requirements of A. terebrans for growth and development. Moisture content loading score on the components was more in PC2 (0.53) with Mg (-0.63) and Ca (-0.48) on the negative side of PC2. This grouping of MC, Mg, and Ca indicates an important factor in A. terebrans nutrition. Correlation analysis also corroborates the positions of major contributors in PC2 with MC negatively correlated with Mg and Ca while Mg and Ca are strongly correlated with each other. Mg and Ca are essential for insect development and play an important role in their physiological and metabolism [28]. Magnesium has been used by humans as a laxative [29] and various sources of the element have been found to increase the moisture content of excreta in birds [30]. Excessive intake of Mg and Ca gets excreted in form of magnesium and calcium carbonate [28] in some insects. Potential shifts identified for A. terebrans were S. mombin and G. arborea which had similar means of magnesium concentration which were the lowest of the selected wood species and both with the lower moisture content of 110.17 ± 5.51 and 80.53 ± 11.30 than the model species (T. mantaly = 111.83 ± 6.74). While high concentration of K is also known for its tolerance role in plants against stress [31], MC~K significant highest relationship for MC~element wise (Table 5) result found in this study suggests that D. regia, T. mantaly, and S. mombin may be more resilient to insect feeding attack than A. indica, G. arborea and T. grandis. These results have a bearing on understanding the feeding behaviours of A. terebrans and factors influencing the selection of healthy living trees as feeding hosts.
Conclusions
While it has been hypothesized that A. terebrans would not shift (H0) or shift (H1) feeding on T. mantaly in the midst of susceptible species, the study shows that there is a shift in feeding preferences of A. terebrans thus supporting the alternative hypothesis (H1). The interplay of MC, Mg, and Ca were major contributors to the second component explaining 26.31% of the variation in the nutritional constituent of the selected wood species. From the wood-preference point of view, S. mombin and/or G. arborea trees may likely be the next shift hosts of A. terebrans in Nigeria if the building up of the population remains unchecked. The highest concentrations of Al, P, S, Fe, and K in S. mombin might be responsible for its highest feeding preference by A. terebrans.
Acknowledgements
The authors are grateful for the support received from Dr. A.T. Oladele of the Department of Forestry and Wildlife Management for his inputs in taking the wood species samples to Centre for Energy Research and Development, Obafemi Awolowo University, Ife for PIXE analysis.
References
- Hill DS, Waller JW (1988) Pests and Diseases of Tropical Crops, 2: 432.
- Agboton C, Onzo A, Quessou Fi, Goergen G, Vidal S, et al. (2014) Insect fauna associated with Anacardium occidentale (Sapindales: Anacardiaceae) in Benin, West Africa. Journal of Insect Science.
- Atuahene SKN (1976) Incidence of Apate spp (Coleoptera: Bostrychidae) on young forest plantation species in Ghana. Ghana Forestry Journal 2: 29-35.
- https://www.fao.org/publications/card/en/c/4ba4b383-f855-5bff-a302-3cbc4bbc3d12/
- De Souza RM, Anjos ND, Mourao SA (2009) Apate terebrans (Pallas) (Coleoptera: Bostrychidae) Atacando Arvores de Nim no Brasil. Neotropical Entomology 38: 437-439.
- Peacock AD (1913) Entomological pests and problems of Southern Nigeria. Bull Entomol Res 4: 191-220.
- Vasconcelos S, Mendes LF, Beja P, Hodgson CJ, Catarino L (2014) New records of insect pest species associated with Cashew, Anacardium occidentale (Anacardiaceae) in Guinea-Bissau. African Entomology 22: 673-677.
- Agboton C, Onzo A, Bokonon-Ganta AH, Tamo M, Vidal S (2017) Breakthrough in the bio-ecology of the cashew wood borer Apate terebrans Pallas (Coleoptera: Bostrichidae), in Northern-Benin. In: Intensification agro-écologique de la production et de la transformation du cajou en Afrique : Problématique, acquis scientifiques et technologiques, perspectives, Côte d'Ivoire 114-125.
- Akanbi MO, Ladipo DO (1988) Azadirachta indica (Neem): Preventing decline and consequent ecological problems in Nigeria through integrated management. In: Dada OB (). Proceedings of 18th Annual Conference of Forestry Association of Nigeria on the role of forestry in combating ecological disasters 55-58.
- Adedeji GA, Zakka U, Aiyeloja AA, Ochuba AI (2018) Response of Terminalia mantaly Perrier wood to beetles tunnelling in Southern Nigeria 17.
- Murray A (1867) XI-list of Coleoptera received from old Calabar on the West Coast of Africa. Annals and Magazine of Natural History 20: 83-95.
- Adedeji GA, Zakka U, Aiyeloja AA, Ayemere CO (2021) Survey of wood borer Apate terebrans tunnels on Terminalia mantaly in Nigeria with special reference to the Niger Delta Region. Journal of Forests 8: 141-152.
- Adedeji GA, Eguakun FS, Egubogo AC, Aiyeloja AA (2022) Insecurities: Special focus on Terminalia mantaly Perrier use in Nigeria 365-370.
- Hasan R, Othman N, Ismail F (2017) Tree species selection in street planting: It’s relationship with issues in urban area. E-BPJ 2: 185-194.
- Omole AO, Moshood AJ (2014) Variations in the wood properties of Terminalia mantaly (H. Perrier) grown as municipal tree in a Nigerian University. Forests and Forest Products Journal 7: 82-89.
- Borowski T (2021) World Inventory of Beetles of the Family Bostrichidae (Coleoptera). Part 2. Check List from 1758 to 2007. World News of Natural Sciences 36: 9-41.
- Oyerinde OV, Olusola JA, Adeoye SA (2018) Assessment of avenue trees species diversity in two selected tertiary educational institutions in Ondo State Nigeria. Journal of Forestry Research and Management 15: 149-167.
- Ancha PU, Ikyaagba ET, Nongov TT (2019) Undergraduate Students Willingness to Pay for Social Services of Trees at the Federal University of Agriculture Makurdi Benue State Nigeria. International Journal of Environment and Climate Change 9: 273-286.
- Eguakun FS, Ishiekwene MC (2021) Comparative study on shading effect of selected avenue trees in University of Port Harcourt, Nigeria. African Journal of Agriculture, Technology and Environment 10: 82-95.
- Fox LR, Morrow PA (1981) Specialization: Species property or local phenomenon? Science 211: 887-893.
- Zhang HJ, Faucher CP, Anderson A, Berna AZ, Trowell S, et al. (2013) Comparisons of Contact Chemoreception and Food Acceptance by Larvae of Polyphagous Helicoverpa armigera and Oligophagous Bombyx mori. J Chem Ecol 39: 1070-1080.
- Conchou L, Anderson P, Birgersson G (2017) Host Plant Species Differentiation in a Polyphagous Moth: Olfaction is enough. J Chem Ecol 43: 794-805.
- Beyaert I, Waeschke N, Scholz A, Varama M, Reinecke A, et al. (2010) Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim Behav 79: 1077-1086.
- Ofodile EAU, Odatuwa-Omagbemi L, Oladele AT, Alade GO (2021) Nutritional and Elemental Characterization of Local Forest Spices used among Itsekiri Ethnics, Nigeria. Journal of Basic and Applied Research in Biomedicine 7: 9-13.
- Highley TL (2004) Wood Formation and Properties Biological Deterioration of Wood. Encyclopedia of Forest Sciences 1846-1851.
- Ulyshen MD, Sobotnik J (2018) An introduction to the diversity, ecology, and conservation of saproxylic insects. Diversity ecology and conservation 47.
- Graziosi I, Tembo M, Kuate J, Muchugi A (2019) Pests and diseases of trees in Africa: A growing continental emergency. Plants People Planet 1-14.
- Clark EW (1958) A review of literature on calcium and magnesium in insects. Annals of the Entomological Society of America51: 142-154.
- Vu MK, Nouwens MA, Biemond I, Lamers CB, Masclee AA (2000) The osmotic laxative magnesium sulphate activates the ileal brake. Alimentary Pharmacology and Therapeutics14: 587-596.
- Van der Hoeven-Hangoor E, Van de Linde IB, Paton ND, Verstegen MWA, Hendriks WH (2013) Effect of different magnesium sources on digesta and excreta moisture content and production performance in broiler chickens. Poultry Science92: 382-391.
- Kabay T (2022) Effects of different potassium doses on growth rates and micronutrients of drought-sensitive beans. J Elemen 27: 239-247.
Citation: Adedeji GA, Aiyeloja AA, Egubogo AC, Amaogu DC, Brown RO (2022) Why Is Wood-Feeder, Apate Terebrans Attacking Only Terminalia Mantaly In The Midst Of Susceptible Urban Tree Species? Evaluation of Wood Feeding Shift Hypotheses. J Plant Sci Curr Res 6: 016.
Copyright: © 2022 Adedeji GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

