
Zinc: Crucial Ion for Male Fertility in the In vitro Reproduction Era
*Corresponding Author(s):
Haim BreitbartThe Mina And Everard Faculty Of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel
Tel:+ 972 35317851,
Email:Haim.Breitbart@biu.ac.il
Abstract
The importance of zinc ion in male fertility was recently proposed in several studies. In the present review we describe the properties, roles and cellular mechanisms of action of Zn2+ in spermatozoa. We focused on the involvement of zinc ion in sperm motility, capacitation and acrosomal exocytosis, three functions that are crucial for successful fertilization. The impact of zinc supplementation on fertilization assisted techniques is also described.
ABBREVIATIONS
AC: Adenylyl Cyclase
sAC: soluble AC
tmAC: trans membrane AC
AE: Acrosomal Exocytosis
EGFR: Epidermal Growth Factor Receptor
ICSI: Intra Cytoplasmis Sperm Injection
GPCR: G-protein Coupled Receptor
HAM: Hyper Activated Motility
IP3: Inositol Triphosphate
IVF: In Vitro Fertilization
NHE: Na+/H+-exchanger
ODF: Outer Dense Fibers
PI3K: Phosphatidylinositol-3-Kinase
PKA: Protein Kinase A
PLC: Phospholipase C
PLD: Phospholipase D
ROS: Reactive Oxygen Species
ZnOPs: Zn Oxide Particles
ZnR: Zn Receptor
ZP: Zona Pellucida
INTRODUCTION
Zinc is an essential element for many biological activities including enzyme regulation, mitochondrial oxidative stress, normal growth, spermatogenesis, digestion and regulation of central nervous system [1]. The concentration of zinc in the body is precisely regulated, and imbalance of zinc would accompany several pathologies including Alzheimer [2-4], cancer, growth retardation, blindness, digestive problems and inflammation [5]. In human body, about 90% of Zn2+ is found in bone and muscle [6]. About 0.1% of bodily Zn2+ is in the serum 60% of it bound to albumin and the remaining to other proteins [7-9]. On the tissue level, 30-40% of Zn2+ is found in the nucleus, 50% in the cytoplasm and the rest in cell membranes [10]. The concentration of Zn2+ in the blood is 3.14mg/l or 4.8mM [11] and in semen ~2mM which is positively correlated with sperm count and normal morphology [12]. Zn2+ deficiency triggers autophagy in yeast [13], which would affect spermatogenesis. It has been shown that Zn2+ supplementation improves serum testosterone levels [14], sperm count [15], plasma membrane and acrosome integrity [16] and restores superoxide antioxidant capacity in asthenospermic men [17]. Interestingly, Zn2+ improves intestinal epithelial barrier function [18] and the integrity of mammary epithelium [19]. Thus it is possible that Zn2+ might enhance the integrity of the epithelium in the capacitation site in the female reproductive tract, resulting in improving sperm fertility. Too high Zn2+ in the dietary to both male and female rats shows significant reduction in fertility [20]. Zn2+ has been linked with key events in the accomplishment of fertilization ability including hyperactivation and acrosomal exocytosis. Defects in sperm quantity, quality and motility account for up to 50% of infertility cases and may affect about 7% of all men [21]. About 25% of infertility cases in human are defined as “unexplained infertility”, and in many cases, a successful fertilization in these men can be achieved by Intra-Cytoplasmic-Sperm-Injection (ICSI) technique. On the other hand, in a not negligible part of these unexplained cases, despite normal sperm quantity, morphology and motility, no egg penetration/fertilization occurs. It is well documented that in order to fertilize, sperm should reside in the female reproductive tract for several hours, in which they undergo a series of biochemical and motility changes collectively called capacitation allowing the spermatozoon to interact with the egg, undergo acrosomal exocytosis and penetration into the egg. Thus it is possible that a significant part of unexplained infertility, that have not been resolved by bypass techniques like ICSI, are in fact caused by spermatozoon failure to performing proper capacitation.
It was shown that zinc deficiency is correlated with a decrease in male fertility [22,23] and zinc in the dietary of dometic animals is required for the achievement of higher fertility rate [24-26]. Sperm mitochondrial sheath [27,28] and sperm chromatin [29,30] are stabilized by zinc bridges. In this review we will focus on the effect of Zn2+ on sperm capacitation, acrosomal exocytosis, including the mechanisms of action and the impact of zinc supplementation on fertilization assisted techniques.
REGULATION OF ZN2+ LEVELS IN THE CELLS
Research in C. elegans identified many genes that were defective in spermatogenesis (spe) and fertilization when mutated [31,32]. For example, spe-8gene that encodes protein tyrosine kinase, involved in protein tyrosine phosphorylation [33,34], a known process that occurs in sperm capacitation [35]. Several proteins function with SPE-8, mediating signaling pathways that promote motility [36,37]. It has been suggested that zinc may initiate SPE-8 signaling cascade leading to sperm activation [38,39]. Working on zinc-transporters revealed that deletion of the homolog zipt-7.1, caused sterility [40]. Zipt-7.1 is a transmembrane protein localized within intracellular organelles [41,42] and together with SPE-8 regulates the release of Zn2+ from internal stores. The released Zn2+ in the cytoplasm activates zinc-regulated proteins that develop motility. Thus, Zn2+ may be considered as second messenger which modulates sperm functions like motility and capacitation. This suggests that intracellular Zn2+ levels should be well controlled by zinc transporters localized in intracellular membranes and in the cell plasma membrane which import Zn2+ from external environment [43].
EFFECT OF ZN2+ ON SPERM CAPACITATION AND ACROSOMAL EXOCYTOSIS
Extracellular zinc had an impact on the intracellular signaling pathway via its interaction with the Zinc Sensing Receptor (ZnR), named also GPR39 [44]. This receptor was found in the sperm acrosome and tail [45-47] suggesting a possible involvement of zinc in sperm functions. We showed that Zn2+ stimulates bovine sperm acrosomal exocytosis [45] as well as Human sperm Hyperactivated Motility (HAM) [46] both mediated by GPR39. The GPR39 belong to GPCR family known to activate the Trans-Membrane-Adenylyl-Cyclase (tmAC). Human sperm treated with 5µM Zn2+ show a 40% increase in intracellular cAMP which is an important event in the capacitation process [45]. It seems that zinc mediates the activity of the two AC isoforms, the sAC as well as the tmAC, leading to intracellular cAMP increase; an effect that was inhibited by the respective specific proteins inhibitors. Surprisingly, the stimulatory effect of extracellular added 8Br-cAMP (a membrane permeabile cAMP analogue) on HAM was also inhibited by sAC inhibitors, conditions by which the cellular levels of cAMP should not be affected [46]. A possible explanation for this result would be that cAMP supplied to the cells is excluded from cellular locations in which sAC provides cAMP for HAM. Interestingly, attempts to bypass the need for sAC activity by providing cAMP did not restore fertilization competence of sAC-null sperm [48]. It has been shown that in vitro addition of high concentration of Zn2+to bovine [45] and human [46] sperm could lead to the inhibition of several capacitation processes and fertility rate [49]. Zinc has antioxidant activity and may decrease Reactive-Oxygen-Species (ROS) levels [16,50]. It was shown that ROS production is essential for sperm capacitation [51,52] however relatively high levels of ROS can harm sperm functions [53]. Thus low zinc concentration might be beneficial in reducing too high levels of ROS, whereas high zinc might decrease ROS to a level that is inhibitory to sperm capacitation. A relatively high concentration of Zn2+ in the millimolar range inhibits human sperm motility [54] and regulates the degradation of semenogelin that prevents capacitation via inhibition of ROS generation [46,55]. This high [Zn2+] also inhibits the voltage-gated H+-channel Hv1, localize in sperm tail and responsible for sperm cytoplasmic alkalization [56,57] and the regulation of human sperm rotation [58]. The cytoplasmic alkalization leads to the activation of the sperm-specific Ca2+-channel CatSper [59] which mediates the development of the capacitation dependent HAM [60]. The stimulatory effect of Zn2+ on human sperm HAM is inhibited by CatSper inhibitor indicating that CatSper mediates Zn2+-stimulated HAM [46]. Zinc enhanced Protein-Kinase A (PKA) activity, Src and Epidermal-Growth-Factor Receptor (EGFR) phosphorylation/activation and these activities are CatSper-dependent [46]. It was shown that Zn2+ is incorporated into sperm ODF extending from the connecting piece of the tail, causing softening of its consistency leading to the development of HAM [61]. In contradiction to the high seminal fluid [Zn2+] (~2mM) which inhibits Hv1, lower concentrations in the micromolar range promote acrosomal reaction in sea urchin [44] and bovine [45] sperm. In sea urchin sperm micromolar Zn2+ activates changes in membrane potential, induce elevation of pHi,[Ca2+] iand cAMP and activate K+-channel [62].
Also, we found that 5-10µM Zn2+ stimulates hyperactivated motility in human sperm incubated under capacitation conditions, whereas at 30µM Zn2+ there is no stimulation [46]. These data clearly show that the relatively high [Zn2+] in the semen are inhibitory to sperm functions, whereas in the female reproductive tract [Zn2+] is much lower (1.0-1.5µM) [63] allowing the occurrence of sperm capacitation/hyperactivated motility and the acrosome reaction leading to fertilization. It has been proposed [64] that Zona Pellucid (ZP) proteinases implicated in endowing the acrosome reacted spermatozoon with the ability to penetrate the ZP, are negatively regulated by Zn2+. It has been shown that sperm can induce Zn2+ release from the oocyte cortex [65,66] leading to proteinases inhibition and as a result sperm that are still bound to the ZP became de-capacitated, and polyspermy is prevented. It was also suggested that Zn2+ inhibits sperm chemoattraction to the egg induced by oocyte-secreted progesterone in human, mouse and rabbit sperm [67]. Addition of Zn2+ (~0.1mM) to bovine IVF medium inhibits fertilization rate [68]. Also, blockers of Zn-dependent metalloproteases inhibit sperm passage via the cumulus ooporus in porcine IVF [69].
Appropriate concentration of zinc, in the micromolar range, seems to increase in vitro capacitation efficiency [45,46] by activating several proteins during this process, including the tyrosine kinase Src, EGFR transactivation and Phosphatidylinositol-3-Kinase (PI3K) [45,70-73] leading to Ca2+ mobilization and acrosome reaction. In a recent study, we suggested the following mechanism that regulates human HAM: Zn2+ stimulates HAM via CatSper-dependent activation of the Adenylyl-Cyclase (AC)/cAMP/PKA/Src/EGFR and Phospholipase C (PLC) cascade [46]. In bovine sperm, we show that Zn2+ activates the EGFR during capacitation which is mediated by activation of tmAC, PKA and Src [45]. The addition of Zn2+ to capacitated bovine sperm further stimulates EGFR and the down-stream effectors PI3K, phospholipase C and protein-kinase C leading to acrosomal exocytosis [45].
ZINC IN ASSISTED REPRODUCTIVE TECHNIQUES
Over the past decade, the efficiency of assisted reproductive techniques has been improved. The cryopreservation of sperm using liquid nitrogen is now usually used in assisted reproduction centers and laboratories as a procedure to preserve sperm cells. However, freezing and thawing processes, cause a decrease of the fertilizing sperm efficiency due to various stress and cryoprotectant toxicity. The sperm is deprived of the seminal plasma protective effects; many antioxidants are stored in human seminal plasma such as vitamin c and e, superoxide dismutase, glutathione and thioredoxin that act directly against free radicals [74,75]. It is well known today that osmotic effects and oxidative stress of cryopreservation affect sperm cells in many ways: by diminishing fertilization capacity, motility, morphology (such as coiled tails), viability of spermatozoa [76] damaging cells membrane [77] causing DNA fragmentation [78,79] and loss of mitochondrial function [80]. The improvement of fertility capacity by certain antioxidants has been more and more used in assisted reproduction techniques [81,82]. The addition of zinc to the culture medium was reported to protect the human spermatozoa from oxidative damage [83]. Studies revealed that after incubation with H202 the DNA fragmentation percentage in spermatozoa was increased (in comparison to control), effect that was reversed by zinc supplementation to the medium [83].
Recent researches brought to light the beneficial effects of zinc addition to human ejaculate before cryopreservation on sperm viability and motility after thawing [83,84]. Freezing of human sperm in the presence of 50µM zinc revealed after thawing a 26%-184% increase in the number of motile sperm and a 130 % increase in the percentage of progressive motility [84]. Similar effects were observed in semen samples cryopreserved with Zinc Oxide Nanoparticles (ZnONPs) after thawing and followed by an incubation of 24h [85]. Moreover when cells were frozen, thawed and refrozen in a second time in the presence of zinc a considerable increase in motility was observed [84]. These significant improvements in sperm motility, when zinc was supplemented to cryopreservation media can be associated with the ion effects on microfilament in the outer dense fiber [86], leading to an increase in sperm mobility percentage [87]. Moreover, zinc has been reported to preserve genomic integrity [88], chromosomal stability [89,90], and protect sperm membrane [91,92] preserving in that way cell morphology during cryopreservation.
Utilization of ZnONPs, used basically as drug delivery for cancer research [93], was applied to study sperm preservation cells during cryopreservation. The ZnONPs seemed to provide beneficial effects by avoiding DNA damages and by stabilizing sperm chromatin [85]. These protecting effects were reported to be linked to the creation of a protective layer of ZnONPs around the sperm cell preventing lipid peroxidation at the membrane [85]. Considerable IVF cases rise from male-factor deficiencies. The quality of sperm after cryopreservation is an essential factor in the success of assisted reproduction procedures. Zinc can be considerate as a good player to this issue (Figure 1).
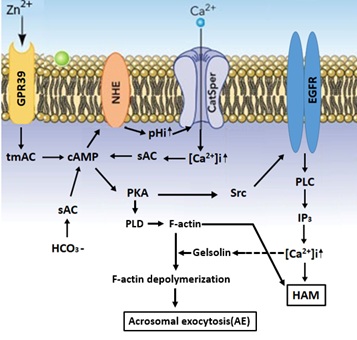
Figure 1: A model describing the mechanisms that mediate the stimulation of HAM and Acrosomal Exocytosis (AE) by Zn2+.
Zn2+ binds and activates GPR39 which activates the tmAC to catalyse cAMP production. NHE (Na+/H+-exchanger) is activated by cAMP leading in increase pHi and activation of CatSper resulting in an increase in [Ca2+] i which together with HCO3- activates sAC. The increase in [cAMP] i causes PKA-activation following by activation of the cascade Src-EGFR-PLC resulting in IP3 production which mobilizes Ca2+ from the acrosome causing further increase in [Ca2+] i and the development of hyper-activated motility. PKA also activates PLD leading to F-actin formation during capacitation. Prior to the AE, Ca2+ activates the actin severing protein gelsolin resulting in F-actin depolymerization and Acrosomal Exocytosis (AE).
REFERENCES
- Giachi G, Pallecchi P, Romualdi A, Ribechini E, Lucejko JJ, et al. (2013) Ingredients of a 2,000-y-old medicine revealed by chemical, mineralogical, and botanical investigations. Proc Natl Acad Sci USA 110: 1193-1196.
- Lovell MA (2009) A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer's disease. J Alzheimers Dis 16: 471-483.
- Szewczyk B (2013) Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 5: 33.
- Prakash A, Bharti K, Majeed ABA (2015) Zinc: Indications in brain disorders. Fundam Clin Pharmacol 29: 131-149.
- Prasad AS (2009) Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12: 646-652.
- Wastney ME, Aamodt RL, Rumble WF, Henkin RI (1986) Kinetic analysis of zinc metabolism and its regulation in normal humans. Am J Physiol 251: 398-408.
- Prasad AS, Oberleas D (1970) Binding of zinc to amino acids and serum proteins in vitro. J Lab Clin Med 76: 416-425.
- Scott BJ, Bradwell AR (1983) Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin Chem 29: 629-633.
- Rukgauer M, Klein J, Kruse-Jarres JD (1997) Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol 11: 92-98.
- Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73: 79-118.
- Li L, Xu G, Shao H, Zhang Z, Pan X, et al. 2017) Analysis of Blood Concentrations of Zinc, Germanium, and Lead and Relevant Environmental Factors in a Population Sample from Shandong Province, China. Int J Environ Res Public Health 14: 227.
- Colagar AH, Marzony ET, Chaichi MJ (2009) Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res 29: 82-88.
- Ding B, Zhong Q (2017) Zinc deficiency: An unexpected trigger for autophagy. J Biol Chem 292: 8531-8532.
- Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ (1996) Zinc status and serum testosterone levels of healthy adults. Nutrition 12: 344-348.
- Underwood EJ, Somers M (1969) Studies of zinc nutrition in sheep. I. The relation of zinc to growth, testicular development, and spermatogenesis in young rams. Aust J Agric Res 20: 889-897.
- Narasimhaiah M, Arunachalam A, Sellappan S, Mayasula VK, Guvvala PR, et al. (2018) Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod Domest Anim 53: 644-654.
- Hadwan MH, Almashhedy LA, Alsalman AR (2015) Oral Zinc Supplementation Restores Superoxide Radical Scavengers to Normal Levels in Spermatozoa of Iraqi Asthenospermic Patients. Int J Vitam Nutr Res 85: 165-173.
- Shao Y, Wolf PG, Guo S, Guo Y, Gaskins HR, et al. (2017) Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J Nutr Biochem 43: 18-26.
- Weng X, Monteiro APA, Guo J, Li C, Orellana RM, et al. (2018) Effects of heat stress and dietary zinc source on performance and mammary epithelial integrity of lactating dairy cows. J Dairy Sci 101: 2617-2630.
- Johnson FO, Gilbreath ET, Ogden L, Graham TC, Gorham S (2011) Reproductive and developmental toxicities of zinc supplemented rats. Reprod Toxicol 31: 134-143.
- Kumar N, Singh AK (2015) Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 8: 191-196.
- Hunt CD, Johnson PE, Herbel J, Mullen LK, (1992) Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Am J Clin Nutr 56: 148-157.
- Zhao J, Dong X, Hu X, Long Z, Wang L, et al. (2016) Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci Rep 6: 22386.
- Nielsen FH (2012) History of zinc in agriculture. Adv Nutr 3: 783-789.
- Kumar N, Verma RP, Singh LP, Varshney VP, Dass RS (2006) Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus x Bos taurus) bulls. Reprod Nutr Dev 46: 663-675.
- Hill GM, Shannon MC (2019) Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol Trace Elem Res 188: 148-159.
- Kerns K, Zigo M, Drobnis EZ, Sutovsky M, Sutovsky P (2018) Zinc ion flux during mammalian sperm capacitation. Nat Commun 9: 2061.
- Song WH, Sutovsky P (2019) Porcine Cell-Free System to Study Mammalian Sperm Mitophagy. Methods Mol Biol 1854: 197-207.
- Roomans GM, Lundevall E, Bjorndahl L, Kvist U (1982) Removal of zinc from subcellular regions of human spermatozoa by EDTA treatment studied by X-ray microanalysis. Int J Androl 5: 478-486.
- Kvist U (1980) Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol Scand 109: 79-84.
- L’Hernault SW, Shakes DC, Ward S (1988) Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 120: 435-452.
- Ward S, Miwa J (1978) Characterization of temperature-sensitive, fertilization-defective mutants of the nematode caenorhabditis elegans. Genetics 88: 285-303.
- Muhlrad PJ, Clark JN, Nasri U, Sullivan NG, LaMunyon CW (2014) SPE-8, a protein-tyrosine kinase, localizes to the spermatid cell membrane through interaction with other members of the SPE-8 group spermatid activation signaling pathway in elegans. BMC Genet 15: 83.
- Hubbard SR, Till JH (2000) Protein tyrosine kinase structure and function. Annu Rev Biochem 69: 373-398.
- Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, et al. (1999) Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod 61: 76-84.
- Geldziler B, Chatterjee I, Singson A (2005) The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev Biol 283: 424-436.
- Nance J, Davis EB, Ward S (2000) spe-29 encodes a small predicted membrane protein required for the initiation of sperm activation in Caenorhabditis elegans. Genetics 156: 1623-1633.
- Shakes DC, Ward S (1989) Initiation of spermiogenesis in elegans: A pharmacological and genetic analysis. Dev Biol 134: 189-200.
- Liu Z, Chen L, Shang Y, Huang P, Miao L (2013) The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development 140: 2103-2107.
- Dietrich N, Schneider DL, Kornfeld K (2017) A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis. Nucleic Acids Res 45: 11658-11672.
- Muhlrad PJ, Ward S (2002) Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics 161: 143-155.
- Arduengo PM, Appleberry OK, Chuang P, L’Hernault SW (1998) The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans J Cell Sci 111: 3645-3654.
- Chu DS (2018) Zinc: A small molecule with a big impact on sperm function. PLoS Biol 16: 2006204.
- Clapper DL, Davis JA, Lamothe PJ, Patton C, Epel D (1985) Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J Cell Biol 100: 1817-1824.
- Michailov Y, Ickowicz D, Breitbart H (2014) Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev Biol 396: 246-255.
- Allouche-Fitoussi D, Bakhshi D, Breitbart H (2019) Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2. Mol Reprod Dev 85: 543-556.
- Schneider M, Forster H, Boersma A, Seiler A, Wehnes H, et al. (2009) Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 23: 3233-3242.
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, et al. (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9: 249-259.
- Abdul-Rasheed OF (2009) The relationship between seminal plasma zinc levels and high molecular weight zinc binding protein and sperm motility in Iraqi infertile men. Saudi Med J 30: 485-489.
- Bray TM, Bettger WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med 8: 281-291.
- Shahar S, Wiser A, Ickowicz D, Lubart R, Shulman A, et al. (2011) Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum Reprod 26: 2274-2282.
- O'Flaherty C, Beconi M, Beorlegui N (1997) Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 29: 269-275.
- Sikka SC (2001) Relative impact of oxidative stress on male reproductive function. Curr Med Chem 8: 851-862.
- Henkel R, Bittner J, Weber R, Huther F, Miska W (1999) Relevance of zinc in human sperm flagella and its relation to motility. Fertil Steril 71: 1138-1143.
- de Lamirande E, Yoshida K, Yoshiike TM, Iwamoto T, Gagnon C (2001) Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J Androl 22: 672-679.
- Lishko P V, Botchkina IL, Fedorenko A, Kirichok Y (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327-337.
- Babcock DF, Rufo GA Jr, Lardy HA (1983) Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc Natl Acad Sci USA 80: 1327-1331.
- Miller MR, Kenny SJ, Mannowetz N, Mansell SA, Wojcik M, et al. (2018) Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep 24: 2606-2613.
- Kirichok Y, Navarro B, Clapham DE (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ Nature 439: 737-740.
- Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, et al. (2014) Structurally Distinct Ca(2+) Signaling Domains of Sperm Flagella Orchestrate Tyrosine Phosphorylation and Motility. Cell 157: 808-822.
- Bolanca I, Obhodas J, Ljiljak D, Matjacic L, Kuna K (2016) Synergetic Effects of K, Ca, Cu and Zn in Human Semen in Relation to Parameters Indicative of Spontaneous Hyperactivation of Spermatozoa. PLoS One 11: 0152445.
- Beltrán C, Rodríguez-Miranda E, Granados-González G, de De la Torre LG, Nishigaki T, et al. (2014) Zn(2+) induces hyperpolarization by activation of a K(+) channel and increases intracellular Ca(2+) and pH in sea urchin spermatozoa. Dev Biol 394: 15-23.
- Ménézo Y, Pluntz L, Chouteau J, Gurgan T, Demirol A, et al. (2011) Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod Biomed Online 22: 647-652.
- Kerns K, Zigo M, Sutovsky P (2018) Zinc: A necessary ion for mammalian sperm fertilization competency. Int J Mol Sci 19: 4097.
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, et al. (2011) Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 6: 716-723.
- Que EL, Duncan FE, Bayer AR, Philips SJ, Roth EW, et al. (2017) Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent Integr Biol (Camb) 9: 135-144.
- Guidobaldi HA, Cubilla M, Moreno A, Molino M V, Bahamondes L, et al. (2017) Sperm chemorepulsion, a supplementary mechanism to regulate fertilization. Hum Reprod 32: 1560-1573.
- Stephenson JL, Brackett BG (1999) Influences of zinc on fertilisation and development of bovine oocytes in vitro. Zygote 7: 195-201.
- Beek J, Nauwynck H, Maes D, van Soom A (2012) Inhibitors of zinc-dependent metalloproteases hinder sperm passage through the cumulus oophorus during porcine fertilization in vitro. Reproduction 144: 687-697.
- Azriel-Tamir H, Sharir H, Schwartz B, Herskfinkel M (2004) Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J Biol Chem 279: 51804-51816.
- Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, et al. (2009) Synaptically-Released Zinc Triggers Metabotropic Signaling via a Zinc Sensing Receptor in the Hippocampus. J Neurosci 29: 2890-2901.
- Chorin E, Vinograd O, Fleidervish I, Gilad D, Herrmann S, et al. (2011) Upregulation of KCC2 Activity by Zinc-Mediated Neurotransmission via the mZnR/GPR39 Receptor. J Neurosci 31: 12916-12926.
- Sharir H, Zinger A, Nevo A, Sekler I, Hershfinkel M (2010) Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J Biol Chem 285: 26097-26106.
- Kobayashi T, Miyazaki T, Natori M, Nozawa S (1991) Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod 6: 987-991.
- Niki E (1991) Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr 54: 1119-1124.
- Nallella KP, Sharma RK, Allamaneni SSR, Aziz N, Agarwal A (2004) Cryopreservation of human spermatozoa: comparison of two cryopreservation methods and three cryoprotectants. Fertil Steril 82: 913-918.
- Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD (1988) Cryopreservation of human spermat III. The effect of cryoprotectants on motility. Fertil Steril 50: 314-320.
- Aitken RJ, De Iuliis GN, McLachlan RI (2009) Biological and clinical significance of DNA damage in the male germ line. Int J Androl 32: 46-56.
- Zribi N, Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, et al. (2010) Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 93: 159-166.
- O’Connell M, McClure N, Lewis SEM (2002) The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 17: 704-709.
- Chow CK (1991) Vitamin E and oxidative stress. Free Radic Biol Med 11: 215-232.
- Donnelly ET, McClure N, Lewis SE (1999) The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 14: 505-512.
- Wu J, Wu S, Xie Y, Wang Z, Wu R, et al. (2015) Zinc protects sperm from being damaged by reactive oxygen species in assisted reproduction techniques. Reprod Biomed Online 30: 334-339.
- Berkovitz A, Allouche-Fitoussi D, Izhakov D, Breitbart H (2018) Cryopreservation of human sperm in the presence of Zn2+ increases the motility rate. J Obstet Gynecol Investig 1: 6-12.
- Isaac AV, Kumari S, Nair R, Urs DR, Salian SR, et al. (2017) Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem Biophys Res Commun 494: 656-662.
- Calvin HI, Hwang FH-F, Wohlrab H (1975) Localization of Zinc in a Dense Fiber-Connecting Piece Fraction of Rat Sperm Tails Analogous Chemically to Hair Keratin. Biol Reprod 13: 228-239.
- Itach SBS, Finklestein M, Etkovitz N, Breitbart H (2012) Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev Biol 362: 154-161.
- Tuerk MJ, Fazel N (2009) Zinc deficiency. Curr Opin Gastroenterol 25: 136-143.
- Blazak WF, Overstreet JW (1982) Instability of nuclear chromatin in the ejaculated spermatozoa of fertile men. J Reprod Fertil 65: 331-339.
- Kotdawala AP, Kumar S, Salian SR, Thankachan P, Govindraj K, et al. (2012) Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet 29: 1447-1453.
- Bettger WJ, O’Dell BL (1981) A critical physiological role of zinc in the structure and function of biomembranes. Life Sci 28: 1425-1438.
- Kendall NR, McMullen S, Green A, Rodway RG (2000) The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim Reprod Sci 62: 277-283.
- Rasmussen JW, Martinez E, Louka P, Wingett DG (2010) Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 7: 1063-1077.
Citation: Allouche-Fitoussi D, Breitbart H (2020) Zinc: Crucial Ion for Male Fertility in the In vitro Reproduction Era. J Reprod Med Gynecol Obstet 5: 034.
Copyright: © 2020 Deborah Allouche-Fitoussi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

