
Long Term Remission of Hyper IgE Syndrome after Treatment with Cyclosporine-A: Clinic and Immunological Correlations
*Corresponding Author(s):
Harb A HarfiDepartment Of Pediatrics And Medicine, National Center Of Allergy, Asthma And Immunology (NCAAI), North King Faisal Hospital And Research Centre, Riyadh, Saudi Arabia
Tel:+966 114803333,
Fax:+ 966 114800480
Email:harfi@allergyarabia.com
Abstract
Three patients, two females and one male, two were brother and sister; their ages were between 10 months and three years. All three patients were suffering from severe eczema, recurrent sinopulmonary infection, lung and skin abscesses, chronically draining ear and failure to thrive since the first few weeks of their lives. Their serum IgE was more than 10 times upper normal for age. Measurement of serum IgE, Cytokine IL-4, and IFN-γ and serum immunoglobulins measure before and after treatment, in addition, skin score of dermatitis and the number of infection, were evaluated before and after treatment with CSA.
Following treatment with CSA, 2-4 mg/kg per day in 2 divided doses, after standard treatment failed. There was a dramatic and sustained clinical improvement, especially dermatitis, associated with marked drop in serum IgE (P<0.0001), IL-4 (P<0.0001), and IFN-γ (P<0.001). There was no significant change in serum levels of IgG, IgA and IgM. The results of our study indicate that immune imbalance in HIE syndrome can be modulated by CSA that leads to marked drop on IgE and IL-4 synthesis and clinical remission. This treatment needs to be repeated in a larger number of patients.
Keywords
Cyclosporin-A; Cytokines; Dermatitis; Hyper IgE syndrome; Immunodeficiency; Interleukin-4
INTRODUCTION
Treatment of HIE patients is frustrating and remains largely unsuccessful. Several treatment modalities have been tried with limited and often temporary success [9-15]. Because HIE patients have excessive production of IgE and IL-4, clinical improvement may be achieved by down regulation of IgE and IL-4. Therefore, we conducted a prospective clinical immunological study to assess the effect of small doses of Cyclosporin-A (CSA) on the clinical course, especially dermatitis and skin infection in HIE syndrome. Three patients with the HIE syndrome showed remarkable clinical improvement following treatment with a small dose of CSA 2-4 mg/kg per day. There was remarkable and significant drop in serum IgE, IL-4 and IFN-γ without affecting immunoglobulin levels after treatment compared to pre-treatment. Our study suggests that the clinical manifestations, especially dermatitis, may be related partially to the high levels of IL-4 and IgE levels which can be modulated by CSA. But these patients have many genetic defect in production of cytokines such as Th17 and IFN-gamma which impairs neutrophils, chemotaxis and IFN-gamma injections reduce the frequency of skin and lung infections [3,16]. Several immunological and pathological abnormalities have been observed in these patients including immunodeficiency markers such as elevated IgD levels, decreased IgG subclasses and poor response to both protein and polysaccharides immunization [16,17]. Some of these patients have symptoms of allergy to foods in 38% and anaphylaxis 8% [18]. These complex immunopathological abnormalities make management of these patients difficult.
MATERIALS AND METHODS
Patients
Scoring of skin inflammation
Routine laboratory investigations
Treatment protocol
Serum IgE, IgG and IgA quantitation
Immunological studies
• Immunophenotyping using Flow Cytometric Cell Analysis (FACS)
• Mitogenic response of PBMC to Phytohemagglutinin (PHA), Pokeweed Mitogen (PWM) and cytokine IL-2
• Natural Killer (NK) cell function
• Cytokine level of IL-α, IL-2, TNF-α, IFN-γ and IL-4
Isolation of PBMC from whole Blood
Mitogens PHA and PWM Induced stimulation of PBMC
(1 μCi/well Sp. activity 6.7 μCi/mM) as given by the β counts of cultures harvested with a cell harvester (Titretek Flow Labs, Rockville, MD) [20].
Interleukin-2 dependent proliferation of PBMC
Natural Killer (NK) cell assay
The percent specific cytotoxicity was computed as follows:
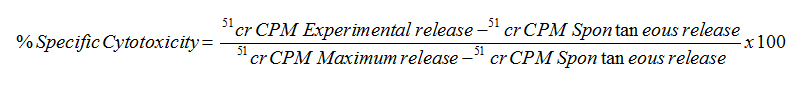
Quantitations of cytokines
Flow Cytometric (FACS) analysis
Statistical evaluation
RESULTS
Patients No 1 and 2 were sister and brother with negative family history of a similar condition. Patient No 3 had 6-year-old maternal cousin who died with identical history. The parents of all our patients (six other patients not reported here) were of consanguine marriage, usually first degree cousins (Figure 1).
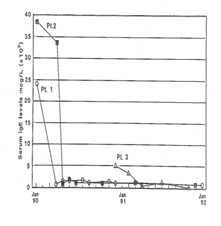
Response to Treatment
|
Serum |
|||||||
|
Patient |
Age |
Sex |
Igs Normal Range |
IgG |
IgA |
IgM |
IgE |
|
1 |
2 years |
F |
Pre-tr. |
3.0 |
0.6 |
0.4 |
24.000a |
|
2 |
10 months |
M |
Pre-tr. |
5.3 |
0.4 |
0.8 |
38.400a |
|
3 |
3 years |
F |
Pre-tr. |
13.2 |
2.6 |
1.8 |
5.040a |
Proliferative response (3HTdR uptake) to mitogens and cytokine
|
Patient |
PHAa |
PWMa |
IL-2b |
|
|
1 |
Pre-treatment |
42453±2363 |
28112±1983 |
49008±3097 |
|
Post-treatment |
63017±2311 |
50307±4763 |
60946±1781 |
|
|
2 |
Pre-treatment |
54583±1932 |
43895±1108 |
54034±3781 |
|
Post-treatment |
64961±1851 |
46388±3192 |
59689±1673 |
|
|
3 |
Pre-treatment |
55257±2117 |
47348±3013 |
54034±1907 |
|
Post-treatment |
59387±3201 |
46407±2011 |
59921±2307 |
|
|
Normal Control (n=10) |
67387±3217 |
49321±2703 |
62831±3781 |
bInterleukin-2 was used at 100 μ/1 x 106/PBL/ml. 105 PBL/well/250 μg were cultured for 72 hours at 37°, 5% CO2 and pulsed with 1 μci3HTdR for 18 hours before harvesting.
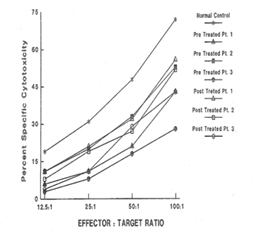
Analysis of Natural Killer (NK) cell function
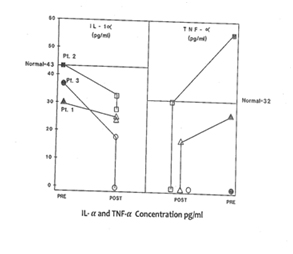
Plasma level of cytokines

Flow cytometry analysis
|
Patients |
Percent Positive cells for various sub-populations of lymphocytes |
|||||||
|
T |
B |
T4 |
T8 |
Ratio |
Act.T |
Nk |
||
|
1 |
Pre-treatment |
70 |
18 |
17 |
44 |
0.3a |
56b |
5c |
|
Post-treatment |
73 |
26 |
23 |
39 |
0.5 |
43 |
7 |
|
|
2 |
Pre-treatment |
68 |
18 |
10 |
57 |
0.2a |
40b |
5c |
|
Post-treatment |
63 |
27 |
42 |
38 |
1.1 |
33 |
8 |
|
|
3 |
Pre-treatment |
50 |
17 |
24 |
31 |
0.8a |
13 |
2c |
|
Post-treatment |
66 |
27 |
36 |
24 |
1.5 |
11 |
7 |
|
|
Normal Control |
78±7 |
16±7 |
39±9.1 |
35±8.7 |
1.1(p?0.05)a |
11±4(p?0.002)b |
16±3(p?0. 02)c |
|
DISCUSSION
In response to low dose (60 - 200 μg/ml Plasma level) CSA treatment, the results of immunoglobulins quantitation showed a remarkable drop in the IgE level in all three patients without any adverse effects of IgM, IgG and IgA immunoglobulins. It is a well accepted phenomenon in mice that subsets of helper T cells, Th1 and Th2, are responsible for differential production of cytokines [40]. Th1 clone when stimulated with appropriate stimulus can produce IL-2, IFN-γ and TNF-α; whereas Th2 clone produces IL-4, IL-5, IL-6, and IL-10. IFN-γ has been shown to be a very important mediator in curtailing the production of IgE, whereas IL-4 molecules support the primary induction and production of IgE [6,41-43]. A similar dichotomy among human T lymphocyte function has been observed in vitro [44]. Our results of flow cytometric analysis are supportive of this notion since absolute number of T and B lymphocytes in HIE patients were not altered, but rather a low T4:T8 ratio was observed in the pre-treated patients. The results of proliferative response to PHA, PWM, and IL-2 indicated inherent defect in either T cell or T cells bearing receptor for IL-2 in HIE patients. The NK cell dependent cytotoxicity in all the three pre-treated patients was substantially low as compared to normal control. An earlier study by Wehrmann et al., demonstrated the number and function of NK cells were altered in atopic patients with high level of circulating IgE [45]. The results of our in vitro study have shown that IgE at higher concentration can decrease regulative killer lineage cells function, implicating similar role of IgE in HIE patients [46]. Our results of impaired PHA response are in disagreement with earlier work. However the response of PWM in HIE patients has been controversial, we found mild to severe suppression of PWM proliferative response prior to CSA treatment with a tendency to improve with CSA treatment [13]. The results of cytokines measurement in our study indicate that IL-1α, TNF-α and IL-2 do not play any significant role in the pathophysiology of HIE Syndrome. Recent reports by Mogensen and Ochs et al., found that susceptibility to infection is related in part to defect in Th17 which results in decreased neutrophil proliferation and chemotaxis decreased inflammation and susceptibility to Candida and bacteria infection [3,47]. The earlier in vitro findings using PMA to stimulate PBMC from HIE patients also showed that inducible IL-2 production is least affected in HIE patients [7]. Our findings of high level of IL-4 and IgE in HIE patients before treatment are confirmatory of previous studies [7]. The most striking findings of this study, after CSA treatment, are that levels of IgE and IL-4 dropped to normal control levels. Following CSA treatment, a sharp decrease to an undetectable level of IFN-γ was observed (P>0.001).
Previous treatment of HIE patients with a variety of agents and immune modulators, including levamisole, cimetidine, transfer factor and ascorbic acid, isotretinoin , cromoglycate and repeated plasma pheresis, failed to induce long term clinical remissions in these patients [9-15,48]. Although interferon-γ was shown in vitro to regulate the IgE production by PBMC form HIE patients, it had no significant clinical effect in treating these patients [41].
Whether this clinical improvement and the normalization of the immune imbalance in HIE patients following treatment with CSA is long lasting and void of serious complication, needs to be evaluated further. After two years of treatment, there is no evidence of any complications except mild hypertrichosis in one patient. On one occasion, the skin lesion flared up three days after CSA was stopped suggesting, at least, that the skin improvement is directly related to CSA therapy.
Recently, low dose cyclosporin-A has been used to treat cases of severe atopic dermatitis with no subsequent serious complications [31,49,50]. Similarly safe and beneficial results after treatment with CSA in a variety of dermatological disorders have been reported [51]. The response of patients with HIE syndrome and severe atopic dermatitis to low dose CSA suggests a common link between these disorders. Both have immune dysregulation, with over production of IgE, severe pruritus and recurrent skin infection with staph aureus. This link needs further investigations in order to uncover possible common mechanism for causing the syndrome.
Though much work is to be done to elucidate the precise mechanism of beneficial effect of CSA treatment in HIE patients, the results discussed here are encouraging for the future use of CSA to treat HIE patients. The effects of a newly and highly immunosuppressive agent FK506 on the IL-4 induced IgE production is also being examined in vitro to find out if it has similar effect as of CSA.
ACKNOWLEDGEMENT
The authors are indebted to Mrs. Eleonor T Barroga for her excellent secretarial assistance in preparing the manuscript.
REFERENCES
- Buckley RH, and Becker WG (1978) Abnormalities in the regulation of human IgE synthesis. Immunol Rev 41: 288-313.
- Woeliner Woellner C, Gertz EM, Schäffer AA, Lagos M, Perro M, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol 125: 424-432.
- Mogensen TH (2013) STAT3 and the Hyper-IgE syndrome: Clinical presentation, genetic origin, pathogenesis, novel findings and remaining uncertainties. JAKSTAT 2: 23435.
- Gernez Y, Freeman AF, Holland SM, Garabedian E, Patel NC, et al. (2018) Autosomal Dominant Hyper-IgE Syndrome in the USIDNET Registry J Allergy Clin Immunol Pract 6: 996-1001.
- Leung DY, Geha RS (1988) Clinical and immunologic aspects of the hyperimmunoglobulin E syndrome. Hematol Oncol Clin North Am 2: 81-100.
- Pene J (1989) Regulatory Role of Cytokines and DC23 in the Human IgE antibody synthesis. Int Arch Allergy Immunol 90: 32-40.
- Vercelli D, Jabara HH, Cunningham-Rundles C, Abrams JS, Lewis DB, et al. (1990) Regulation of Immunoglobulin (Ig)E Synthesis in the Hyper-IgE Syndrome. J Clin Invest 85: 1666-1671.
- Alsum Z, Hawwari A, Alsmadi O, Al-Hissi S, Borrero E, et al. (2013) Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty-five patients. J Clin Immunol 33: 55-67.
- Businco L, Laurenti F, Rossi P, Galli E, Aiuti F (1981) A child with atopic features raise serum IgE, and recurrent infection treated with levamisole. Arch Dis Child 56: 60-63.
- Friedenberg WR, Marx JJ Jr, Hansen RL, Haselby RC (1979) Hyperimmunoglobulin E Syndrome: Response to transfer factor and ascorbic acid therapy. Clin Immunol Immunopathol 12: 132-136.
- Mawhinney H, Killen M, Fleming WA, Roy AD (1980) The hyperimmunoglobulin E syndrome--A neutrophil chemotactic defect reversible by histamine H2 receptor blockade? Clin Immunol Immunopathol 17: 483-491.
- Leung DYM, Wood NL, Geha RS (1985) Reversal of cellular abnormalities in the hyper IgE syndrome following plasmapheresis. Clin Res 33: 161A.
- Geha RS, Leung DYM (1989) Hyper Immunoglobulin E Syndrome. Immunodef. Rev 1: 155-172.
- Shuttleworth D, Holt PJA, Mathews N (1988) Hyper Immunoglobulin E syndrome: treatment with isotretinoin. Br J Dermatol 119: 93-99.
- Yokota S, Mitsuda T, Shimizu H, Ibe M, Matsuyama S (1990) Cromoglycate treatment of patient with hyperimmunoglobulinaemia E syndrome. Lancet 335: 857-858.
- Buckley RH (2001) The hyper-IgE syndrome. Clin Rev Allergy Immunol 20: 139-154.
- Sheerin KA, Buckley RH (1991) Antibody responses to protein, polysaccharide, and phi X174 antigens in the hyperimmunoglobulinemia E (hyper-IgE) syndrome. J Allergy Clin Immunol 87: 803-811.
- Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, et al. (2013) Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol 132: 1388-1396.
- Boyum A (1968) Isolation of mononuclear cells and granulocytes by one step centrifugation and of granulocytes by combining centrifugation and sedimentation at Ig. Scand J Clin Lab Invest 97: 77-98.
- Kwaasi AA, Parhar RS, Harfi H, Tipirneni P, al-Sedairy ST (1992) Characterization of antigens and allergens of date palm (Phoenix dactylifera) pollen. Immunological assessment of atopic patients using whole extract or it fraction. Allergy 47: 535-44.
- Parhar RS, Yagel S, Lala PK (1989) PGE2-mediated immunosuppression by first trimested human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol 120: 61-74.
- Parhar RS, Ernst P, Sheth KV, al-Sedairy S (1992) Anti-Tumor cytotoxic potential and effect on human bone marrow GM-CFU of human LAK cells generated in response to various cytokines. Eur Cytokine Netw 3: 299-306.
- Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S (1991) Endotoxemia and release of Tumournecrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol 70: 2640-2644.
- Borel JF, Feurer C, Mag?ee C, Stähelin H (1977) Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology 32: 1017-1025.
- Calne RY, White DJ, Evans DB, Thiru S, Henderson RG (1981) Cyclosporin A in cadaveric organ transplantation. Br Med J (Clin Res Ed) 282: 934-936.
- Calne RY, Rolles K, White DJ, Thiru S, Evans DB, et al. (1979) Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 2: 1033-1036.
- Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schröter GP (1981) Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med 305: 266-269.
- Kahan BD (1989) Cyclosporine. The New England Journal of Medicine 321: 1725-1738.
- Biren CA, Barr RJ (1986) Dermatologic applications of cyclosporine. Arch Dermato. 122: 1028-1032.
- Griffiths CE, Powles AV, Leonard JN, Fry L, Baker BS, et al. (1986) Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed) 293: 731-732.
- Van Joost T, Stolz E, Meule F (1987) Efficacy of low dose cyclosporine in severe atopic skin disease. Arch Dermatol 123: 166-167.
- Britton S, Palacios R (1982) Cyclosporin A-Useful, risks and mechanism of action. Immunol Rev 65: 5-22.
- Bunjes D, Hardt C, Röllinghoff M, Wagner H (1981) Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol 11: 657-661.
- Reem GH, Cook LA, Vilcek J (1983) Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science 221: 63-65.
- Wang SC, Zeevi A, Jordan ML, Simmons RL, Tweardy DJ (1991) FK 506, rapamycin, and cyclosporine: effects on IL-4 and IL-10 mRNA levels in a T-helper 2 cell line. Transplant Proc 23: 2920-2922.
- DeKruff RH, Turner T, Abrams JS, Palladine MA, Umetsu DT (1989) Induction of human IgE synthesis by CD4+ T cell clones. Requirement for interleukin 4 and low molecular weight B cell growth factor. J Exp Med 170: 1477-1494.
- Ishizaka A, Sakiyama Y, Nakanishi M, Tomizawa K, Oshika E, et al. (1990) The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol 79: 392-396.
- Rousset F, Robert J, Andary M, Bonnin JP, Souillet G, et al. (1991) Shifts in interleukin-4 and interferon-gamma production by T cells of patients with elevated serum IgE levels and the modulatory effects of these lymphokines on spontaneous IgE synthesis. J Allergy Clin Immunol 87: 58-69.
- Romagnani S, Maggi E, Del Prete G, Parronchi P, Tiri A, et al. (1989) Role of interleukins in induction and regulation of human IgE. Clin Exp Rheumatol 7: 117-122.
- Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, et al. (1988) The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev 102: 5-28.
- King CL, Gallin JI, Malech HL, Abramson SL, Nutman TB (1989) Regulation of immunoglobulin production in hyperimmunoglobulin E recurrent-infection syndrome by interferon gamma. Proc Natl Acad Sci USA 86: 10085-10089.
- Ishizaka A, Joh K, Shibata R, Wagatsuma Y, Nakanishi M, et al. (1990) Regulation of IgE and IgG4 synthesis in patients with hyper IgE syndrome. Immunology 70: 414-416.
- Gauchat JF, Lebman DA, Coffman RL, Gascan H, de Vries JE (1990) Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med 172: 463-473.
- Del Prete G, Maggi E, Parronchi P, Chrétien I, Tiri A, et al. (1988) IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their J Immunol. 140: 4193-4198.
- Wehrmann W, Reinhold U, Kuekl S, Franke N, Uerlich M, et al. (1990) Selective Alternation in natural killer cell subsets in patients with atopic dermatitis. Int Arch Allergy Appl. Immunol 92: 318-322.
- Parhar RS, Ernst P, Al-Mohanna F, Kwaasi A, Sheth KV, et al. (1991) 425 In vitro regulation of immune cell functions by human IgE. J Allergy and Clinical Immunol 87: 2-246.
- Ochs HD, Oukka M, Torgerson TR (2009) TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol 123: 977-983.
- Donabedian H, Alling DW, Gallin JI (1982) Levamisole is inferior to placebo in the hyperimmunoglobulin E recurrent-infection (Job's) syndrome. N Engl J Med 307: 290-292.
- Logan RA, Camp RD (1988) Severe atopic eczema: response to oral cyclosporin A. J R Soc Med 81: 417-418.
- Taylor 3rd, Cooper KD, Headingto JT, Ho VC, Ellis CN, et al. (1989) Cyclosporine therapy for severe atopic dermatitis. J Am Acad Dermatol 21: 580-583.
- Ho VC, Lui H, McLean DI (1990) Cyclosporine in nonpsoriatic dermatoses. J Am Acad Dermatol 23: 1248-1259.
Citation: Harfi HA, Parhar RS, al-Sedairy S (2019) Long Term Remission of Hyper IgE Syndrome after Treatment with Cyclosporine-a: Clinic and Immunological Correlations. J Allergy Disord Ther 5: 009.
Copyright: © 2019 Harb A Harfi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

