Abbreviations
Ac-DEVD-AFC: acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin
C: solution with the cellobiose synthesized in the laboratory
Ctrl: cells grown only in DMEM medium without being treated with the plant extract, fraction of cellobiose or cellobiose itself
DMEM: Dulbecco’s Modified Eagle’s Medium
DTT: Dithiothreitol
F: Fraction with cellobiose, sulphur, vitamin C and selenium isolated from the plant extract
FBS: Fetal Bovine Serum
MTS: 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
KDMEM: Knock out Dulbecco’s Modified Eagle's Medium
PBS: Phosphate Buffered Saline
RIPA: Radio-Immunoprecipitation Assay buffer
7-AAD: 7-Amino-Actinomycine
VC: Vitamin C
V-PE annexin: annexin V
R: phycoerythrin conjugate
S: Sulphur
Se: Selenium
Z-VAD-FMK: N-benzyloxycarbonyl-Val-Ala Asp (OMe)-fluoromethylketone
Introduction
Fetomaternal hemorrhage was first described by Wiener in 1948 [1]. This condition is considered physiological if a small amount of fetal blood enters the maternal circulation [1] and it occurs in 50 to 75% of all pregnancies. In this case, the volume of the transfusion ranges from 1 to 50 mL. The majority of blood losses are 1 mL or less, 1 in 400 cases are approximately 30 mL and 1 in 2000 the transfusion volume is about 100 mL [2].
Sometimes, besides the rarity of the condition, FMH involves a large amount of fetal blood which can lead to important fetal morbidity and mortality [1]. During pregnancy, clinical manifestations can be subtle and nonspecific which difficult the recognition of this condition. Antenatal suspicion of the diagnosis should occur when absent fetal movements is reported.
We describe this case to alert for this condition and the importance of maternal symptoms and newborn clinical findings. The prompt recognition and intervention is the key to the prognosis of this entity that can be fatal.
Clinical Case Report
Cancer is an uncontrolled growth of abnormal cells. It is found in animals as well as humans. There are many different types of cancer that are found in animals, symptoms are often similar to those in people (eg., abnormal swelling, unexplained weight loss etc.,). Cancer is one of the leading causes of death in companion animals such as dogs and cats; it is particularly common in animals that live 10 years or longer. If treatment is appropriate this may include chemotherapy and surgery or radiotherapy [1].
Evasion of apoptosis is a hallmark of cancer. To date, several pathways for activation of caspases and apoptosis have been identified including the death receptor pathway mediated by caspase 8, the mitochondrial pathway mediated by cytochrome-c release and caspase 9 activation [1-4].
Cell death is followed by cell elimination. In multicellular animals, cell elimination is essential for development and homeostasis, and is mainly carried out by apoptosis. Historically, apoptosis defined a type of cell death that was not only morphologically distinct from cell death but also possessed distinct biochemical and molecular features [1].
A novel class of pro-apoptotic agents being investigated as potential therapeutics for the treatment of cancer and other malignancies are sugars. We are therefore entering the era of biologically targeted therapies, where also monosaccharides and disaccharides together with other molecules play an interesting attempt to cancer treatment [2,5-8]. Previous in vitro studies have confirmed that exposure of human endothelial cells to high glucose induces significant cell death via apoptosis [5]. Documents reported that apoptosis is particularly prominent in models of hyperglycemia injury; however the mechanism and clinical complications still remain unclear [5,9].
To predict toxicity, corrosivity, and other safety variables as well as the effectiveness of new medicines for humans, traditional testing of chemicals, consumer products, medical devices and new drugs has involved the use of animals. But today, scientists have developed and validated alternative methods shown to lead to safer and more effective products and drugs for humans than animal testing [10-12].
The present studies therefore exploit the options of treatment of cancer with molecules of natural origin acting in synergistic effect, in our case cellobiose complex including cellobiose, vitamin C, sulphur and selenium. The study aims to elucidate the pivotal apoptotic and cell death role of cellobiose complex and those natural compounds that contain cellobiose complex and its related sugars. It also sheds some light into the mechanism of action of cellobiose complex and cellobiose itself in different types of animals cells such as MEF wt and immortalized MEF cells, but not only this, since the present study correlates this data to a new computer method of virtual testing [5,11-13]. It investigates the efficiency of chosen molecules and therefore offering new insights into the effectiveness of substances and potential side effects of new medicines without using animal experiments [5,14,15].
Materials and Methods
Materials
In our research we used the following materials: MTS from Promega (USA), Bradford reagent from Bio-Rad (Germany), Z-VAD-FMK, Ac-DEVD-AFC, DTT from Bachem (Switzerland), FBS from Gibco (USA), antibiotics Penicillium, streptomycin and glutamax from Gibco (USA), annexin V-PE and 7-AAD for flow cytometry were purchased from BD Biosciences (USA). Fluorescent organelle-specific probe MitoTracker CMXRos was of Molecular Probes (Eugene, OR, USA) and cellobiose, vitamin C, sulphur and selenium were from Calbiochem Germany. All other reagents (RIPA buffer, caspase buffer, KDMEM, Tryple Select and PBS) were prepared at the institute according to biochemical procedures as previously described [5,15].
Cell lines
The cell lines used in the experiments were mouse embryonic fibroblasts MEF wt and MEF wt imm (ATCC). Before performing experiments the cells were tested for the cell line identification by isozyme typing and karyotyping. The cells were last tested one month before we started the experiments. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 20% Bovine Serum (FBS), 1% Penicilium/streptomycin and 1% glutamax grown to obtain 90% confluency. When cells were confluent and prepared for experiment with the plant extract, they were transferred in 96-well or 6-well plate, where they were grown in DMEM, free of any kind of supplement of animal origin.
Preparation of plant extract
Capsicum chinense and Allium neapolitanum were grown in a greenhouse. Once the plants grown in the greenhouse blossomed and produced first fruits, the whole plant was collected and dried. Our calculation was as follows: 100g of Alium sativum contained 1g cellobiose, 31.2 mg vitamin C, 14.2 µg of selenium and 10 µg of sulphur, while 100g of Capsicum chinense contained 76.4 mg of vitamin C, 8.8 µg of selenium and 36.6 g of cellobiose [15-20]. Dry plants were ground into a powder. The powder was suspended in DMEM to which Penicillium and streptomycin were added, together with glutamax (1% v/v each of antibiotic and glutamax in the final mixture). The mixture was left at room temperature for 3 days and shaken several times in between. After 3 days the extract was filtered and by these means ready for the experiments.
Cell culture
All cells were grown in the 10 cm plates (N=3) to obtain 90% confluency and afterwards washed with 1×PBS and treated with tryple select 5-10 minutes (depending on the cell line). In the next step the cells were transferred into 96-well (N=1 for 96-well plate, each concentration in 96 well-plate was represented by N=3 repeats) and 6-well plates (N=1 for 6-well plate, each concentration in 96 well-plate was represented by N=3 repeats) at a ratio of 10000 cells/well (MEF wt and MEF wt imm) and grown overnight. The next day the cells were treated with the plant extract or with the solution of cellobiose itself in the concentration range of 0.00l-1000 mg/mL (raising concentrations by a factor of 10; N=3 for each concentration) and incubated for 48 hours (depending on the cell line and the onset of apoptosis). We used two control cells. The first were grown only in DMEM (N=3) and the other ones in DMEM with Penicillium and streptomicinum in a 1% v/v (N=3). All experiments were repeated 3 times. The results show only control cells grown in DMEM.
Determination of cell death
MTS assay: The first analysis of cytotoxicity was measured using MTS assay. Cells were cultured at 1×106 in 6-well plates (N=3) overnight before treatment with the plant extract at final concentration (N=3 for each concentration), depending on the cell line. For the comparative control experiments the cells were incubated overnight only in DMEM. After incubation with the plant extract, specific for each cell line, the cells were observed by light microscopy (Olympus I×71, Japan, magnification 40 and 60). To prepare the extracts, cells were collected in three parallels, pelleted by centrifugation at 1000 rpm for 5 minutes and washed twice with 1× PBS. Whole-cell extracts were prepared in RIPA buffer (50 mM Tris, pH 8.0, 100 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Nonidet P-40 0.5% w/v deoxycholic acid, 1mM EDTA) in three parallels. Following 10 minutes incubation on ice, the insoluble material was removed by centrifugation at 14000 rpm for 10 min. Cytosolic extracts were prepared as previously described [15]. Total protein concentration was determined using the Bradford assay.
The first impression of apoptosis was measured by the addition of 20 µL of MTS into 100 µL samples in the 96-well plate (N=3 for each concentration). After 45 minutes incubation we measured the absorbance at 490 nm with a 96-well plate reader (TECAN). For further analysis we chose the concentrations of plant extracts that induced 20-50% cell death and in the next step we measured caspase activity in these samples (N=3).
Caspase (DEVDase) activation determination: Cells were cultured at 0.5×106cells/ml in 6-well plates (N=3) in 2 mL DMEM per well overnight before various treatment with either the plant extract (at 0.001-10 mg/mL), solution of pure cellobiose (at 10-1000 mg/mL) or with cellobiose in the combination with added vitamin C, sulphur and selenium (0.00l-1000 mg/mL) and incubated for 24 hours. To prepare the cell extracts for caspase activity detection, cells were collected in three parallels, pelleted by centrifugation at 1000 rpm for 5 minutes and washed twice with PBS. Whole-cell extracts were prepared in 35 µl RIPA buffer (50 mM Tris, pH 8.0, 100 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Nonidet P-40 0.5% w/v deoxycholic acid, 1mM EDTA) in three parallels. After 7 minutes of incubation on ice cells were again subjected to centrifugation this time 5 minutes on 14000 rpm. In white 96-well-plate we transferred 40 µl of each samples and added 50 µl of 2X caspase buffer/1M DTT and 10 µl of DEVD substrate. We gathered 40 µg of protein from cells, untreated and treated with the plant extract in the presence or absence of inhibitor Z-VAD-FMK, to determine caspase activity and by measuring the proteolytic cleavage of the fluorogenic substrate Ac-DEVD-AFC (Bachem) has also shown in standard protocols [4]. Before absorbance measurement the plate was incubated at 370C in the incubator with 5% CO2. The absorbance was measured by TECAN xfloor4 program at 400-505 nm and DEVDase activity calculated in RFU [2,9,15].
Apoptosis detection by Annexin V and 7-AAD staining: Early apoptotic and dead cells were quantified by flow cytometry measurements of phosphatidylserine exposure and 7-AAD incorporation. The cells were grown in the 10 cm Petri dishes (N=3) to obtain 90% confluency and afterwards washed with PBS and treated with tryple select 5-10 minutes (depending on the cell line). In the next step the cells were transferred into 6-well plate (N=1 for 6-well plate, each concentration in 6 well-plate was represented by N=3 repeats), where the cells were treated with either the plant extract in the concentration range 0.001 mg/mL-10 mg/mL, the solution of pure cellobiose in the concentration range 10-1000 mg/mL or with cellobiose in the combination with added vitamin C, sulphur and selenium in the range of 0.00l-1000 mg/mL and incubated for 24 hours. Cells were washed twice with cold PBS and then resuspended in 1X binding buffer at a concentration 1x106 cells/mL. Briefly, 100 µL aliquots of cells were labelled with 5 µl annexin V-PE and 5 µl 7-AAD according to the manufacturer’s instructions and transferred into 5 mL culture for each concentration of plant extract, pure cellobiose or combination of molecules separately. The cells were gently vortexed and incubated for 15 minutes at room temperature (25oC) in the dark. In the end 400 µl of 1X binding buffer was added to each tube. The cells were then subjected to flow cytometry analysis using FACScalibur flow cytometer (BD Biosciences) and analyzed with the CellQuest software within one hour.
Determination of apoptotic pathway: To determine by which apoptotic pathway (intrinsic and extrinsic) the plant extract triggers apoptosis we used MitoTracker Red CMXRos to assess and monitor the integrity of mitochondria. The cells were grown in the 10 cm Petri dishes (N=3) to obtain 90% confluency and afterwards washed with PBS and treated with tryple select 5-10 minutes (depending on the cell line). In the next step the cells were transferred into 6-well plate (N=1 for 96-well plate, each concentration in 6 well-plate was represented by N=3 repeats). The cells were treated with either the plant extract in the concentration range 0.001 mg/mL-10 mg/mL, the solution of pure cellobiose in the concentration range 10-1000 mg/mL or with cellobiose in the combination with added vitamin C, sulphur and selenium in the range of 0.00l-1000 mg/mL (raising concentrations by a factor of 10; N=3 for each concentration) and incubated for 24 hours. The cells were washed twice with PBS and then stained with MitoTracker CMXRos, which was added to the cells at a final concentration of 20 nM. Following 30 minutes incubation at 37°C the cells were washed in PBS and again re-suspended in PBS for the measurement on a flow cytometer. Afterwards we measured red fluorescence of 5000 or 10000 cells (depends on the chosen cell line) per sample, corresponding to mitochondria using the FL3 channel on a flow cytometer.
Virtual animal and human model
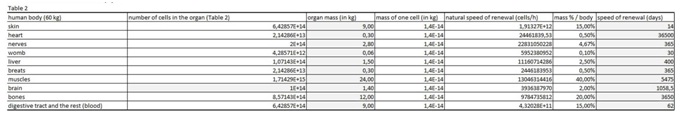
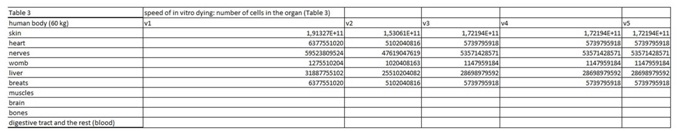
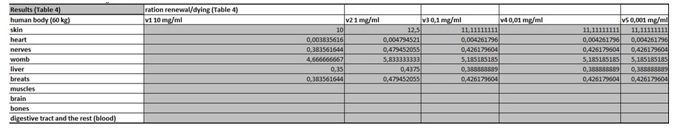
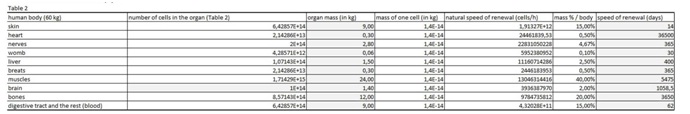
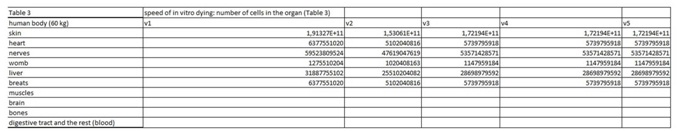
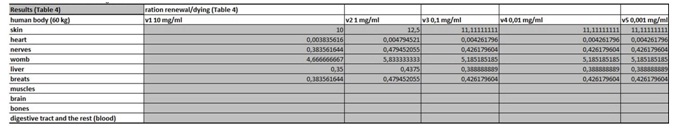
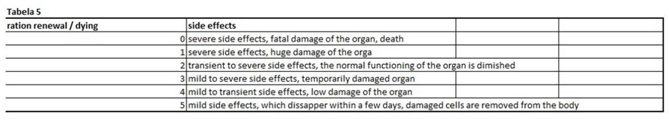
The present virtual and human model is based on the in vitro results of apoptosis, which are then calculated on the whole mass of the particular organ and in the end on the body mass of the organism. The model is divided in five parts (Figures 1 and 2 (table 1-5)). In the first spreadsheet the results of the in vitro tests are entered: cell type (MEF wt and immortalized MEF wt), final number of cells in the confluent growth, time of incubation with the tested substance and finally the percentage of dead cells for each concentration and cell type (the concentration range from 1 µg/mL to 10 mg/mL). Under columns v1-v5 we have calculated the speed of dying cells (number of cells in apoptosis multiplied with the final number of cells and divided with the time of incubation). In the second spreadsheet we have entered some data from the literature. It is known how many cells are there in the human brain and the mass of individual organ in the human body. According to this data we have calculated number of cells in each organ (for example the brain there are 1014 cells and the mass is 1.4 kg, this is then correlated with the body organ with different mass for example skin has 9 kg and this means there are 9 kg×1014 cells/1.4 kg = 6.2×1014 cells in the skin). From this data we can then calculate the mass of one cell (mass of the organ divided with the number of cells in the organ). When we calculate the natural speed of cells renewal, we divide the number of cells in the organ with the speed of renewal of the cells calculated per hour. In the tables 1-5 (Figures 1) we show the calculation, where we multiply the number of cells in the organ with the corresponding speed of in vitro dying of the cells. The result we then divide with the number of cells in vitro of the corresponding cell line. This we calculate for each speed, cell line and organ (see markings from v1 to v5). In the tables 1-5 (Figure 1) we calculate the ratio between the rate of renewal and dying of the cells, which is in continuation defined in the tables 1-5 (Figure 1) (for example result range 0-1 means the huge damage of cells, fatal damage of the organ and possible death; as the number rises this means the side effect are not so severe and therefore the chances of renewal of the body and the elimination of damaged cells are much higher). The same model can be then adjusted to any other model in our case we have matched the data for laboratory mouse.
Programming of the virtual animal and human model
We have programmed the virtual animal and human model firstly with the existing chemotherapeutics that are already in use. We have used dosage regimen of oxaliplatin, which served us as control. Once we got the results from it we entered the data of the plant extract and cellobiose complex with sulphur, selenium and vitamin C. According to the acquired data we then concluded the efficacy and potential of cellobiose complex and plant extract to be considered for further experiments in the process of cancer drug evaluation.
analysis and statistics
The percentage of apoptotic and dead cells were reported as mean ± standard error. Data were analyzed using the two-way Analysis Of Variance (ANOVA). Differences between experimental and control groups and among different exposure groups were regarded as statistically significant at the level of *p<0.05, **p< 0.001 and *** p < 0.0001.
Results
The effect of the plant extract Solanaceae on MEF wt and immortalized cells
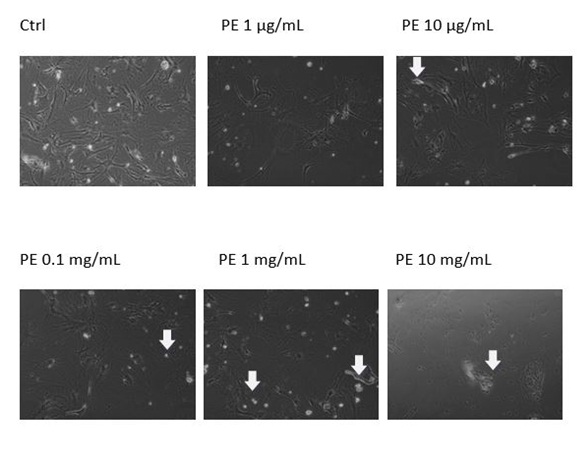
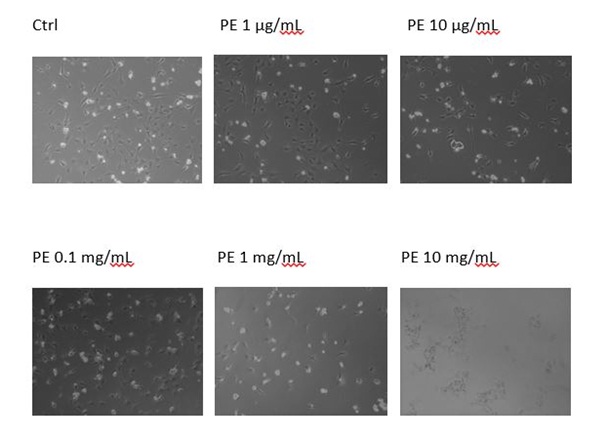
In order to determine whether the plant extract Solanaceae induced growth inhibition due to programmed cell death, we evaluated the apoptotic indices at the indicated times of treatment (i.e., 48h) by microscopical analysis. We discovered that the plant extract had no effect on MEF wt cells, but some apoptotic morphology was visible in immortalized MEF wt cells with the concentrations range from 10 mg/mL to 1 µg/mL. In immortalized MEF wt cells there was some cell death morphology visible together with the debris of the cells (Figures 3A and B).
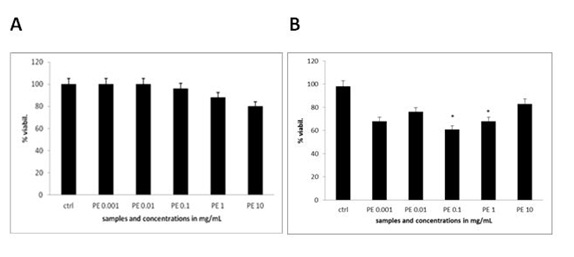
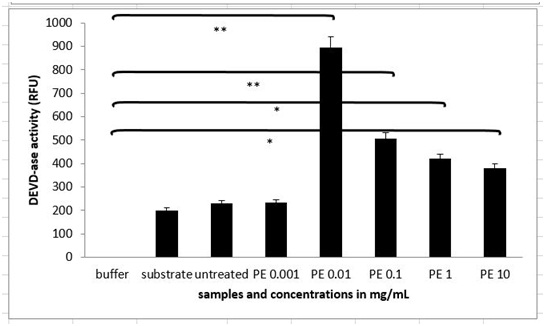
The extent of apoptosis was quantified by MTS test, where there was no significant change in the viability of MEF wt cells across the concentration range of the plant extract from 1 µg/mL to 10 mg/Ml (Figure 4A). When treating the immortalized MEF wt cells with the plant extract there was a significant loss of viability (Figure 4B). For example concentration of the plant extract 0.1 mg/mL induced cell apoptosis in 23.10% cells (N=3 repeats and parallels; SEM + 16.24; P < 0.05), while the concentration of the plant extract 1 mg/mL induced apoptosis in 18.77 % (N=3 repeats and parallels; SEM + 14.73; P < 0.05) as shown in figure 4B. As we wanted to determine whether caspase-dependent apoptotic pathways were involved, we performed the DEVDase activity assay. We discovered that only when the cells were treated with the plant extract of 0.01 mg/mL (N=3 repeats and parallels; 831.33 RFU (SEM + 72.47; P < 0.05) and 0.1 mg/mL (N=3 repeats and parallels; 548.67 RFU (SEM + 145.06; P < 0.05), there was then highest significant activation of caspase-3. The results were in correlation with MTS test (Figure 5). When we studied the integrity of mitochondria, we found out there was no loss of integrity, when treating the cells with the plant extract in the concentration range of 1 mg/mL to 1 µg/mL nor with the pure cellobiose in the same concentration range (data not shown).
Results
The effect of the plant extract Solanaceae on MEF wt and immortalized cells
In order to determine whether the plant extract Solanaceae induced growth inhibition due to programmed cell death, we evaluated the apoptotic indices at the indicated times of treatment (i.e., 48h) by microscopical analysis. We discovered that the plant extract had no effect on MEF wt cells, but some apoptotic morphology was visible in immortalized MEF wt cells with the concentrations range from 10 mg/mL to 1 µg/mL. In immortalized MEF wt cells there was some cell death morphology visible together with the debris of the cells (Figures 3A and B).
The extent of apoptosis was quantified by MTS test, where there was no significant change in the viability of MEF wt cells across the concentration range of the plant extract from 1 µg/mL to 10 mg/Ml (Figure 4A). When treating the immortalized MEF wt cells with the plant extract there was a significant loss of viability (Figure 4B). For example concentration of the plant extract 0.1 mg/mL induced cell apoptosis in 23.10% cells (N=3 repeats and parallels; SEM + 16.24; P < 0.05), while the concentration of the plant extract 1 mg/mL induced apoptosis in 18.77 % (N=3 repeats and parallels; SEM + 14.73; P < 0.05) as shown in figure 4B. As we wanted to determine whether caspase-dependent apoptotic pathways were involved, we performed the DEVDase activity assay. We discovered that only when the cells were treated with the plant extract of 0.01 mg/mL (N=3 repeats and parallels; 831.33 RFU (SEM + 72.47; P < 0.05) and 0.1 mg/mL (N=3 repeats and parallels; 548.67 RFU (SEM + 145.06; P < 0.05), there was then highest significant activation of caspase-3. The results were in correlation with MTS test (Figure 5). When we studied the integrity of mitochondria, we found out there was no loss of integrity, when treating the cells with the plant extract in the concentration range of 1 mg/mL to 1 µg/mL nor with the pure cellobiose in the same concentration range (data not shown).
The effect of stable HPLC fraction containing cellobiose, sulphur, selenium and vitamin C
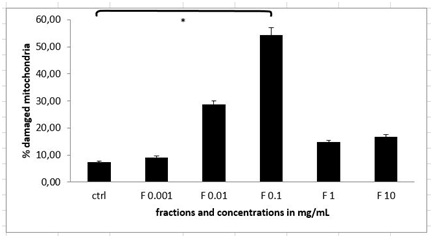
Since we wanted to discover, whether there are any molecules in the plant extract that could trigger apoptosis and cell death, we prepared the stable HPLC fraction of the combination of two cold plant extracts of Alium sativum and Capsicum chinense as previously described [5,15,21]. When treating the immortalized MEF wt cells with the different concentrations of fractions (concentration range from 1 µg/mL to 10 mg/mL), we discovered that with the fraction with the concentration of 0.1 mg/mL there were 52.97% of mitochondria (N=3 repeats and parallels; SEM + 6.31; P < 0.05), which lost their integrity (Figure 6).
Pure solution of cellobiose and its effect on MEF wt and immortalized cells
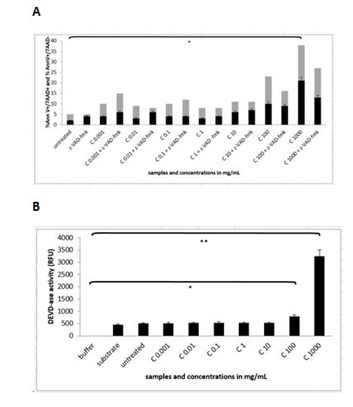
The results with the flow cytometry measurements showed that only concentration with pure cellobiose of 1000 mg/mL induced statistically significant rate of apoptosis and necrosis (N=3 repeats and parallels; 24.67, SEM + 4.06; P < 0.05). However Z-VAD-FMK did not rescue the cells from cells death in any of the concentrations of pure cellobiose from 0.001 up 1000 mg/mL (Figure 7A).
Measurements of DEVDase activation showed some different insights into the molecular mechanism of apoptotic and necrotic process, since there was the highest activation of caspase-3 up to 3.012 RFU (N=3 repeats and parallels; SEM + 239.24; P < 0.05) only in the cells treated with the solution of pure cellobiose in the concentration of 1000 mg/mL (Figure 7B).
The effect of unique complex cellobiose/sulphur/selenium/vitamin C
Since we discovered there was not the same effect on the cells with pure cellobiose in contrast to plant extract, we studied the plant extract furtherly with NMR analysis and discovered there are other molecules present in the HPLC fraction as well [10]. According to our previous researches on human cancer cells HepG2, we discovered it was the cellobiose together with vitamin C, sulphur and selenium responsible for the cell death. We therefore repeated the experiments with the same combination of molecules [10].
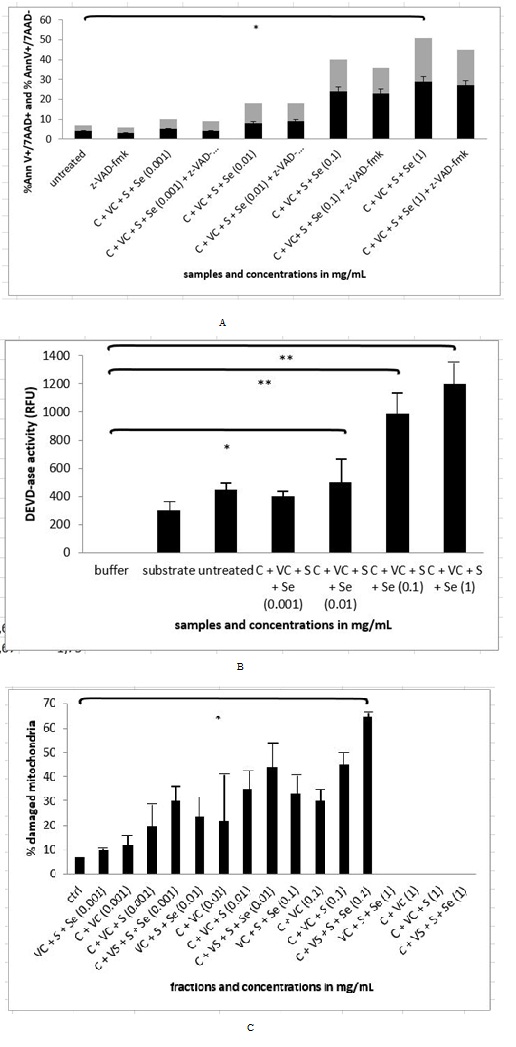
We managed to detect 21.80 % of apoptotic cells (N=3 repeats and parallels; SEM + 2.05; P < 0.05) and 26.47% of necrotic cells (N=3 repeats and parallels; SEM + 8.08; p < 0.05), when treating the cells with the cellobiose complex in the final concentration of 1 mg/mL (Figure 8A). When studying the activation of caspase-3 enzyme (Figure 8B) with the cellobiose complex (cellobiose, vitamin C, sulphur and selenium), we discovered the statistically significant activation of 395.67 RFU in concentration of 0.01 mg/mL (N=3 repeats and parallels; SEM + 94.66; P < 0.05) in concentration of 0.1 mg/mL 571.67 (N=3 repeats and parallels; SEM + 187.09; P < 0.001) and 852.00 RFU in concentration of 1 mg/mL (N=3 repeats and parallels; SEM + 120.08; P < 0.001). While treating the cells with the pan-caspase inhibitor Z-VAD-FMK it was seen that the blockade was not achieved in any concentration, suggesting the apoptotic pathway was triggered also through non-caspase dependent apoptotic pathway (Figure 8B). Concerning the loss of mitochondria integrity, there was a significant change only with the concentration 0.1 mg/mL of cellobiose complex, where 63.10% (N=3 repeats and parallels; SEM + 7.83; P < 0.05) of mitochondria loss their integrity, while higher concentrations 1 mg/mL and 10 mg/mL were leading into cell debris and we were therefore unable to detect anything (Figure 8C).
Experiments in virtual animal and human model
In order to show how the virtual model is operating, we performed several experiments. In animal and human virtual model we entered the data from the in vitro experiments with the plant extract containing cellobiose, sulphur, selenium and vitamin C (Figures 1 and 2). Here only the results in human virtual model are shown since we used oxaliplation as control, which is used for the treatment of cancer in human. We have discovered that if we treat the cells with high concentrations of medicines and cells suddenly, the human organism cannot survive. This was clearly seen also with low dosages, if the percentage of dead cells was high in a short time frame. The dosage regimen of oxaliplatin (Figure 1) clearly demonstrates, that if we treat the human organism in longer time span (14 days) with the dosage of 0.7 mg/mL - 1.0 mg/mL, where we continuously and slowly induce only 1% of apoptotic or damaged cells, the chances of organism to survive and eliminate cancer and damaged cells is extremely high. Finally the concentrations of cellobiose complex (Figure 2) obtained in in vitro tests showed us very promising results especially for skin cancer and adenocarcinoma, where the chances of survival and renewal of the human organism were in correlation with the results obtained with the virtual model testing with oxaliplatin (Eloxatin). However cellobiose complex (cellobiose, vitamin C, sulphur and selenium) was shown not to be effective for other cancer types, since the results show too high damage of cells and therefore low chances of survival.
Discussion
Sugars derived from natural products are a promising class of medicines and are being developed for therapeutic use for the treatment of cancer. The mechanism of action of sugars as anti-cancer agents is complex and not yet fully understood as also shown by previous studies Naha et al., and Yang et al., [2,9]. As we reported in our previous articles, sugars especially cellobiose induces cell death by promoting the loss of mitochondrial integrity thus leading to the complex process of cell death [5,15]. We are the group focusing on the effect of cellobiose complex (cellobiose, vitamin C, sulphur and selenium) isolated from the plant extract Capsicum chinense and Allium sativum in comparison to cellobiose synthesized in the laboratory and to the unique molecular complex of cellobiose, sulphur, selenium and vitamin C [5,15].
Whether plant extract and solution of cellobiose induced PCD, we next evaluated the rate of apoptosis and cell death in immortalized MEF wt cells and in MEF wt cells. In metazoans apoptosis is a major physiological process of cell elimination during development and in tissue homeostasis and can be involved in pathological situations [1].
According to our data there is a difference between immortalized and non-immortalized-primary cells, since we have observed the plant extract had no effect on MEF wt cells, except in the concentration of 10 mg/mL, where there was a loss of viability for 20% (Figures 1-4).
In our study the sequence of events initiated by plant extract and later on solution of cellobiose itself was explored. We studied how immortalized MEF wt and primary MEF wt cells reacted, when we treated them with the plant extract and solution of cellobiose. We demonstrated there was a different response to plant extract with cellobiose complex (cellobiose, sulphur, selenium and vitamin C) and to solution of cellobiose in immortalized MEF wt cells in contrast to primary MEF wt cells, where there was no significant effect, suggesting there were some differences in the receptors in the cells membrane, by which molecules could enter the cell, what was also previously explored by Pelengaris et al., [1]. In immortalized MEF wt cells we observed there was a dose response to plant extract in higher concentrations from 0.1 mg/mL to 1 mg/mL, but this was not in linear correlation with the selected dosage range in the plant extract, where much lower concentrations were needed for the same effect of apoptosis and cell death [4,5,15,22]. For example the concentration of the plant extract of 1 µg/mL induced 28.10% of apoptosis in immortalized MEF wt cells, while 1.000x higher concentration of the mentioned extract induced 18.77% of apoptosis due to the fact that in higher concentrations the cells underwent cell death or simply bursted in the process (Figure 6) [15]. While treating the cells with pure solution of cellobiose, this did not show any effect in the same concentration range, since much higher concentrations (1000 mg/mL) were needed, which resulted in bursting of the selected immortalized cells (Figure 7). It is therefore crucial to understand that the optimal treatment with the natural compounds can be also in animal model achieved only with the isolation of optimal combination of molecules, what was shown in the experiment of studying the rate of apoptosis and cell death by the combination of molecules celobiose/Se/S/vitamin C (Figure 8A). In this stable HPLC fraction there were cellobiose, vitamin C, sulphur and selenium present, which together acted as efficient in triggering programmed cell death in lower dosages. However when studying whether the caspases were activated (Figure 8B), we noticed that efficient caspase activation (P < 0.05) was achieved only with the concentration of 1 mg/mL and 10 mg/mL of plant extract and from 100 to 1000 mg/mL of pure solution of cellobiose (P < 0.001), suggesting there was also other apoptotic and cell death pathway involved as clearly shown by Deeb et al., and Bakshi et al., [4,23]. These data were in correlation with the results of the experiment with the pan-caspase inhibitor Z-VAD-FMK, which was not able to rescue the cells from apoptosis or cell death in any of the above mentioned concentrations [5,15]. As we investigated what happens with the mitochondria, we discovered that here 52.97% of mitochondria were damaged in immortalized MEF wt cells (Figure 6), while treating the cells with the active HPLC fraction of cellobiose complex (cellobiose, vitamin C, sulphur and selenium) of the concentration of 0.1 mg/mL, isolated from the plant extract with the HPLC method using hypercarb colon, what was comparable when treating the cells with the cocktail of cellobiose/Se/S/vitamin C (Figure 8C) [15]. This was another confirmation that, when triggering apoptosis and cell death via described molecule complex this involves intrinsic apoptotic pathway as well.
In this study we additionally confirmed correlation to already observe cytotoxicity of high concentration of sugars especially glucose and cellobiose as previously demonstrated by Yang et al., [9]. This was also indicated in our case, where apoptosis (26.47%) and necrosis (23.87%) was determined in immortalized MEF wt cells with the concentration of cellobiose/Se/S/vitamin C of 1 mg/mL with flow cytometry analysis. There are common mechanisms of the mode of action of cellobiose complex in human as well as in animal immortalized and cancer cells, suggesting there are some agents that can be commonly used for the treatment of cancer in human as well as in animal model.
As exploring the mode of action of the virtual animal and human model, we have discovered one important fact [10,14]. If we treat the cells with high concentrations of medicines within short time frame of 24 to 48 hours, up to 35% of cells die. This percentage is too high and the organism cannot survive. This was clearly seen also with low dosages, if the percentage of dead cells was high in a short time frame. When used the chemotherapeutics that are in use for longer periods, we have discovered in the case of oxaliplatin (Figure 1), which was considered as control, that if we treat the organism in longer time span (14 days) with the dosage of 0.7 mg/mL-1.0 mg/mL, where we continuously and slowly induce only 1% of apoptotic or damaged cells, the chances of organism to survive and eliminate cancer and other damaged cells is extremely high. What we were most interested were the results with cellobiose complex, since we have proven that the plant extract was too aggressive and caused too high percentage of dead cells in determined time span of 24 to 48 hours (Figure 2). It was interesting to observe the results with cellobiose complex were in correlation with the results obtained with the virtual model testing with oxaliplatin. The concentrations of cellobiose complex obtained in in vitro tests showed us very promising results especially for skin cancer and adenocarcinoma, where there was low but continuos rate of apoptosis present together with the range of side effects, which were mild enough for organism to survive and to eliminate damaged and cancerous cells.
Conclusion
Taken together it was clearly shown there are clear differences in effect of the plant extract, pure solution of cellobiose, the active HPLC fraction and cellobiose complex including cellobiose, vitamin C, sulphur and selenium on the rate of onset of apoptosis and cell death in immortalized MEF wt and primary MEF wt cells. The optimal therapeutic response can be achieved only with the synergistic combination of selected active molecules, proven to induce apoptosis and cell death of target immortalized cells. The mode of action of apoptosis and cell death induced by cellobiose, vitamin C, sulphur and selenium shows mitochondria as being the main target resulting in the loss of their outer membrane potential leading the cells via intrinsic apoptotic pathway to cell death. Compared to the treatment with oxaliplatin virtual human model showed us that cellobiose complex showed comparable results as oxaliplatin inducing low but steady rate of apoptosis differing only in the concentration range.
Figures
Figure 1: The tables 1-5 comprises the figure 1 which explains virtual human model for the testing of new medicines and substances. As a control dosage regimen of oxaliplatin (Eloxatin) was used.
Table 1: In the first spreadsheet the results of the in vitro tests are entered: cell type, final number of cells in the confluent growth, time of incubation with the tested substance and finally the percentage of dead cells for each concentration and cell type. In this figure 1 we entered in vitro data on oxaliplatin.
Table 2: In this table we entered data from the literature on the cell mass of individual organ.
Table 3: In this part of the figure 1 we show the calculation, where we multiply the number of cells in the organ with the corresponding speed of in vitro dying of the cells. The result we then divide with the number of cells in vitro of the corresponding cell line. This we calculate for each speed, cell line and organ (see markings from v1 to v5).
Table 4: In this table we calculate the ratio between the rate of renewal and dying of the cells.
Figure 5: The last table shows us potential side effects and their ratio of severity.
Figure 2: Which comprises table 1-5 which explains virtual human model for the testing of new medicines and substances. Here the data obtained from the in vitro experiments with cellobiose complex containing cellobiose, selenium, sulphur and vitamin C were entered into the model.
Table 1: In the first spreadsheet the results of the in vitro tests are entered: cell type, final number of cells in the confluent growth, time of incubation with the tested substance and finally the percentage of dead cells for each concentration and cell type. In this figure 2 we entered in vitro data on our molecules complex of cellobiose, selenium, sulphur and vitamin C.
Table 2: In this table we entered data from the literature on the cell mass of individual organ.
Table 3: In this part of the Figure 1 we show the calculation, where we multiply the number of cells in the organ with the corresponding speed of in vitro dying of the cells. The result we then divide with the number of cells in vitro of the corresponding cell line. This we calculate for each speed, cell line and organ (see markings from v1 to v5).
Table 4: In this table we calculate the ratio between the rate of renewal and dying of the cells.
Table 5: The last table shows us potential side effects and their ratio of severity.
Figure 3A: Apoptotic morphology in MEF wt imm (A) and in MEF wt cells.
Figure 3B: Apoptotic morphology in MEF wt imm (B). In both types of cells we observe the morphology of cells treated with the plant extract (marked as PE) in concentration range 10 mg/mL-1 µg/mL.
Figure 4A and B: Viability of MEF wt and MEF wt imm cells determined by MTS test after the treatment with the plant extract (marked as PE). Error bars denote mean ¬+ SEM. *P < 0.05; ** P < 0.001.
Figure 5: DEVDase activity in MEF wt imm cells after treatment with the plant extract (marked as PE). Error bars denote mean ¬+ SEM. *P < 0.05; ** P < 0.001.
Figure 6: Integrity of mitochondria in MEF wt imm cells after the treatment with the stable HPLC fraction containing cellobiose, sulphur, selenium and vitamin C (marked as F). Error bars denote mean ¬+ SEM. *P < 0.05; ** P < 0.001.
Figure 7A and B: Flow cytometry analysis and measurement of DEVDase activity in MEF wt imm cells after treatment with the pure solution of cellobiose (marked as C). Error bars denote mean ¬+ SEM. ** P < 0.001.
Figure 8A-C: Flow cytometry, measurement of DEVD-as activity and mitochondrial integrity in MEF wt imm cells after treatment with Cellobiose (C), Sulphur (S), Selenium (Se) and Vitamin C (VC). Error bars denote mean ¬+ SEM. *P < 0.05; ** P < 0.001.