ABSTRACT
Background: Hereditary Hemochromatosis (HH) can lead to complications including cirrhosis, diabetes and osteoporosis and pituitary dysfunction. The recommended initial treatment is phlebotomy adjusted based on ferritin levels. The extent to which HH affects the quality of life is not known.
Aim: To evaluate the quality of life patients with HH.
Methods: We included all patients with genetically verified HH undergoing phlebotomy at four clinical sites in Denmark. Evidence of complications was systematically assessed. Quality of life was assessed using the Short Form 36 (SF-36) questionnaire. Predictors of the quality of life (SF-36 total score) were evaluated in univariable and multivariable regression analysis.
Results: Twenty five patients (median age 60 years; 13 men) were included. The median number of times patients underwent phlebotomy in the year before inclusion was 6 (range 4-8). Four patients had impaired glucose tolerance, two had diabetes, nine had a borderline response to the Synacthen test and two had evidence of hypogonadotropic hypogonadism. In multivariable regression analyses, only ferritin was a significant predictor of the quality of life (P=0.031).
Conclusion: This study found that a low ferritin may be having a detrimental effect on the quality of life in patients with HH. Based on the small sample size, we cannot make any definite conclusions. The results suggest that the quality of life may be considered when evaluating treatment goals, e.g., in the elderly with a low risk of complications, but additional research is needed to evaluate our findings.
KEYWORDS
Hereditary disease; Iron overload related disease; Liver disease; Serum ferritin; Transferrin saturation.
Abbreviations
HH: Hereditary Hemochromatosis
SF-36: Short Form 36
IGF-1: Insulin-like Growth Factor-
TSH: Thyroid Stimulating Hormone
T4: Thyroxine
T3: Triiodothyronine
LH: Luteinizing Hormone
SHBG: Sex Hormone Binding Globulin
FSH: Follicle-Stimulating Hormone
OGTT: Oral Glucose Tolerance Test
SEM: Standard Error of the Mean
Introduction
Hereditary Hemochromatosis (HH) is a genetic disorder resulting in iron overload. The HFE-gene related subtype 1 is the most common cause of HH in Northern Europe. The inheritance is autosomal recessive and penetrance is between 13.5 and 50%. About 80-90% of patients with HH have a mutation in C282Y on the HFE-gene resulting in hepcidin dysfunction [1-3]. In HH, dysfunctional hepcidin leads to increased iron uptake in the duodenum via ferroportin and increased release of iron from deposits in macrophages [4]. The iron accumulates in several organs including the liver, pancreas, pituitary glands, heart and skin. In hereditary hemochromatosis phlebotomy is recommended to remove the excess iron that is toxic to cells. Increased iron deposits in the liver and pancreas may lead to cirrhosis, hypogonadotropic hypogonadism and diabetes. The frequency of endocrine insufficiencies in HH is debated [5-9]. A study including patients with HH related cirrhosis found endocrine dysfunction in nine of nine patients. However, a more recent study found that 5% of women and 6% of men with HH had Hypogonadotropic hypogonadism and a similar study found that only one patient out of 22 (5%) had a borderline gonadotropic deficient [9,10]. However, there are no studies evaluating the influence of pituitary dysfunction and the quality of life in patients with HH [11]. A cross sectional study found that pituitary dysfunction due to surgery or chemotherapy lead to reduced quality of life. Pituitary dysfunction may have a similar impact on the quality of life in patients with HH [12]. Therefore, we evaluated the association between the quality of life, ferritin and pituitary hormones in patients with HH. Ferritin levels are used to evaluate the treatment with a target level of 50 to 100 µg/L. Alleviating the iron overload is believed to decrease the risk of complications including diabetes, osteoporosis and cirrhosis. It may also reduce the risk of pituitary dysfunction. Whether phlebotomy improves health related quality of life is debated [13,14-16].
Materials and Methods
Patients
We recruited all consecutive adult patients with genetically verified HH undergoing phlebotomy from the outpatient clinics at four Danish University Hospitals (Aalborg University Hospital and Copenhagen University Hospitals Hvidovre, Gentofte and Koege) from 2010 to 2011. Our inclusion criteria were age > 18 years, a verified mutation on the HFE gene (C282Y and H63D homozygote’s). Patients with other possible causes of iron overload e.g., secondary iron overload due to transfusion or erythropoiesis disorders, excessive alcohol intake (>24 g alcohol/day for women and >36g alcohol/day for men) or ongoing infections were excluded.
Ethics
All patients gave their written informed consent according to the declaration of Helsinki. Scientific Ethics Committee Capital Region of Denmark and the Danish data protection agency approved the study.
Outcomes
The primary outcome was quality of life assessed by the Short Form 36 questionnaire (SF-36). SF-36 is an international validated questionnaire used to evaluate the general state of health. The questionnaire uncovers emotional wellbeing, role limitations due to emotional and physical health problems, physical functioning, bodily pain, social functioning, energy or fatigue and general health perceptions. Each parameter is scored from 0 (worst well-being) to 100 (best well-being) [17].
Participant characteristics and laboratory assessments
We registered the baseline characteristics of included participants based on patient history and conducted a clinical and biochemical evaluation. Baseline data included age, gender, year of diagnosis, age at diagnosis, number of phlebotomy, known HH-related complications, comorbidities and the Charlson Comorbidity Index. Laboratory assessments included serum ferritin, hemoglobin, serum iron, transferrin saturation and liver blood tests [18]. Pituitary function was evaluated by Insulin-like Growth Factor-1 (IGF-1), Thyroid Stimulating Hormone (TSH), Thyroxine (T4), Triiodothyronine (T3), prolactin, Luteinizing Hormone (LH) and Sex Hormone Binding Globulin (SHBG). We measured Follicle-Stimulating Hormone (FSH) and estrogen in female patients and testosterone in male patients. All tests were performed using hospital standard analysis and the results of patients were evaluated individually.
Patients underwent an Adrenocorticotropic Hormone (ACTH) test with Synacthen (250 mg tetracosactide, synthetic corticotropin). We defined a normal response as plasma cortisol levels >550 nmol/L and an increase of >250 nmol/L, 30 minutes after Synacthen injection and a borderline response as values between 400 -549 nmol/L. Glucose tolerance was tested by an Oral Glucose Tolerance Test (OGTT). We defined diabetes as an OGTT with blood glucose levels >11 mmol/L after two hours, while patients with glucose values between 7.9 and 11.0 mmol/L were classed as having impaired glucose tolerance. Assessment of the bone mineral density was performed by Dual-energy X-ray Absorptiometry (DXA) scan [19].
We evaluated the health-related quality of life using the Short Form 36 (SF-36) questionnaire. The SF-36 is an internationally validated questionnaire used to evaluate the general state of health. The questionnaire uncovers emotional well-being, role limitations due to emotional problems, role limitation due to physical health problems, physical functioning, bodily pain, social functioning, energy/fatigue and general health perceptions [20].
Statistics
The statistical analyses were performed in STATA version 14 (STATA Corp, Texas, USA). Patient characteristics were summarized using medians with range for continuous variables or proportions for binary variables. Our analyses evaluated the potential predictors in univariable and multivariable linear regression analyses with backward elimination. To evaluate the influence of having a low ferritin level, we generated an indicator variable to compare the quality of life in patients with values below the 30th centile (<60 mg/L) or higher than the 30th centile (≥60 mg/L). The regression models included the following variables: ferritin (indicator variable as described), known complications to HH (cirrhosis, diabetes, heart disease or osteopenia), normal/abnormal pituitary hormones, years since diagnosis, age, gender and Charlson Comorbidity Index (CCI).
Results
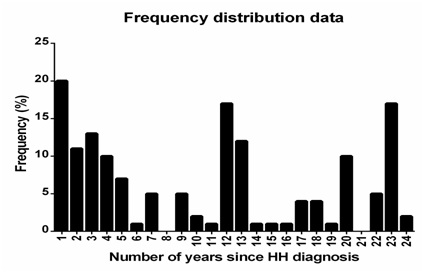
We identified 27 potentially eligible patients. Two declined to participate in the study. Twenty-five patients (one with H63D and 24 with C282Y mutation) agreed to participate and were included in our analyses (Figure 1).
The median age was 60 years (range 30 to 79 years) and median number of years since the diagnosis was made was 5 (range 1 to 20 years). Thirteen patients (52%) were men. The ferritin level ranged from 16 to 1020 mg/L and the hemoglobin levels from 5.7 to 10.3 mmol/L. Two patients had hemoglobin below the normal range 5.7 and 6.9 mmol/l. Both patients were women and had ferritin levels above 60 mg/L (100 and 690 mg/L, respectively). The hemoglobin levels of patients with ferritin levels above or below the 30th percentile (60 mg/L) were not significantly different (P=0.95). Only a small number of patients had co-morbidities. Accordingly, 19 (79%) had a Charlson Comorbidity Index (CCI) of zero, four scored 1 point (17%) and one scored 5 points (4%). Three patients had congestive heart disease (12%), one had cirrhosis (4%) and one had osteopenia (4%). Four patients had impaired glucose tolerance (16%) and two had diabetes (8%). All patients underwent phlebotomy during the previous year. The median number of times patients underwent phlebotomy was 13 (range 3 to 27) for patients diagnosed with HH within five years and four (range 0 to 8) for patients diagnosed with HH more than five years before inclusion in the study. None were treated with Desferal (Table 1).
Six patients (24%) had a border line peak response to the Synachten test and three (12%) had a low increase. The remaining patients had a normal response. Four patients (16%) had impaired glucose tolerance and two (8%) had diabetes. Three patients had low IGF-1 (12%) and two had low prolactin levels (8%). None had clinical evidence of thyroid disease. Among male patients, one (4%) had high LH (13 IU/L) but normal testosterone and SHBG. None had hypogonadotropic hypogonadism. Among female patients, two post-menopausal women had low LH and FSH levels (8%). Both had normal (low) oestradiol levels, indicating hypogonadotropic hypogonadism. None had signs of osteoporosis, but one patient had osteopenia. For further details, please see supplementary table 1.
Quality of life
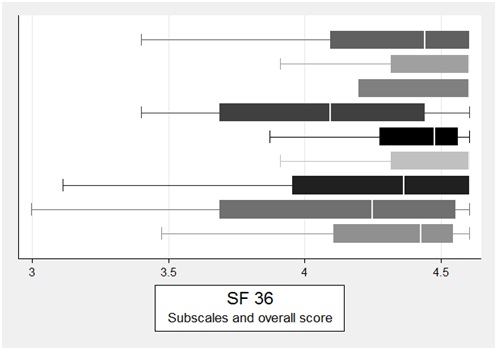
The median total SF-36 score was 82.81 (63.97-92.98) (Figure 2).
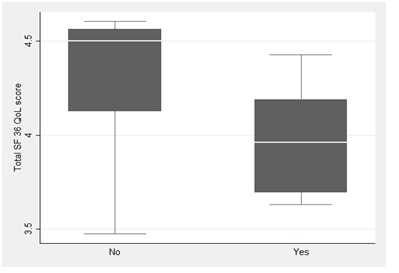
There were no differences between the total scores of patients with a normal or abnormal Synacthen test (P=0.163), glucose tolerance (P=0.859), or IGF-1 (P=0.523) [21,22]. In the univariable linear regression analysis, serum ferritin below 60 mg/L was a significant predictor of the quality of life (regression coefficient β 0.28; standard error [SE] 0.12; P=0.031) (Figure 3).
None of the remaining covariates were significant in the univariable analysis (hemoglobin (P=0.993), borderline response to the synacten test P=0.326; low IGF-1 levels P=0.954; impaired glucose tolerance P=0.283; HH related complications P=0.081). After including all variables in the model, ferritin level below 60 mg/L was the only significant predictor of the quality of life.
Discussion
This study found that there may be an association between lower ferritin levels and a lower health-related quality of life in patients with HH undergoing phlebotomy. Based on our limited sample size, we cannot make any definite conclusions. It is possible that clinically relevant predictors of the quality of life were overlooked. However, our results could suggest that the quality of life could be considered when making treatment goals. Additional evidence is needed to confirm our findings before using our results in clinical practice. Future trials could evaluate the potential detrimental effects of phlebotomy on quality of life and the trade-off between treatment and the risk of complications especially in groups who may theoretically have a lower risk of complications (such as the elderly).
Our study only includes 25 patients and analyses found that ferritin levels below 60 mg/L and HH-related complications were associated with a lower score on the SF-36 questionnaire. In multivariable analyses, only the ferritin level remained statistically significant. Based on our sample size, we are unable to make any definite conclusions regarding ferritin and the quality of life. In Denmark, phlebotomy is adjusted based on ferritin levels. Based on the current availability of diagnostic tests and reduced costs associated with genetic testing. Patients with HH may therefore be diagnosed earlier and therefore undergo treatment for several years. It therefore seems relevant to evaluate the potential effect of ferritin on the quality of life and the potential trade-off between treatment and the risk of developing HH-related complications.
We are unable to address causal relationships and explain why ferritin correlates with lower SF-36 scores. We do not have long term follow up and were unable to determine the influence of reducing the increased iron levels through phlebotomy. For a young patient, achieving ferritin levels between 50 or 100 mg/L reduces the risk of HH related complications. In theory, the risk of developing HH related complications could be less important than the risk of reducing the quality of life in elderly patients. The oldest patient included in our study was 79 years and 25% were older than 69 years of age. Our findings suggest that the quality of life is a factor that may be considered when setting the therapeutic goal for patients in this age category.
It is likely that certain subgroups of patients with HH-related complications have an impaired quality of life. We found that the quality of life scores were lower in patients with HH related complications, but the association was not significant in multivariable analyses after adjusting for ferritin levels. In addition, we were unable to show differences between patients with specific complications. This could possibly reflect limited statistical power.
Previous studies have found that diabetes and hypogonadotropic hypogonadism are the most common endocrine complications to HH. We only identified two patients (8%) with hypogonadotropic hypogonadism, which is low compared to other studies [23-26]. Likewise, only 16% had impaired glucose tolerance (age ranged 65-79) and 8% had diabetes. The prevalence of diabetes in the Danish population is 5% (all age groups) and 17% have impaired glucose tolerance (age group 60-70 years) [27,28]. Thus, the prevalence of diabetes and impaired glucose tolerance in our patients with HH is similar to the Danish population in general [29]. We included several patients who had been diagnosed with HH at an early age. Theoretically, our findings could reflect a beneficial effect of phlebotomy or that included patients had been diagnosed early (at a non-advanced stage). Unlike previous studies, we included few patients with cirrhosis or diabetes. The fact that a large proportion of patients in previous studies had cirrhosis or diabetes suggests that they were diagnosed at an advanced disease stage and may therefore have a higher rate of complication to HH [23-25].
The prevalence of corticotroph insufficiency in HH is debated. We found nine patients without a normal response in the Synacthen test [11,30]. We were surprised to find that the Synacthen test result was not associated with the quality of life scores. Based on our results, additional prospective studies evaluating the quality of life and measuring the Synacthen response after 30 and 60 minutes in patients with HH seem relevant. However, at present, regular screening of patients does not seem warranted.
Conclusion
We found that ferritin levels below 60 mg/L are associated with a poorer quality of life. Additional studies are needed to evaluate the extent to which the quality of life may be included in assessments of patients and treatment goals.
Figures
Figure 1: Frequency plot showing the years since the HH diagnosis.
Figure 2: Quality of life of included patients assessed using the SF-36 questionnaire; total score and the sub scores General Health Perception, Bodily Pain, Social Functioning, Emotional Wellbeing, Energy/Fatigue, Emotional Limitations, Role Limitations Due to Physical Functioning and Physical Functioning.
Figure 3: Quality of life (SF-36 total score) of patients with ferritin <60 ml/L or ≥60 mg/L. The figure (box plot) shows the median, interquartile range and total range.