Abbreviations
Tricuspid Valve Atresia ((TVAtr))
Congenital Heart Defect (CHD)
Right Ventricular (RV)
Right Atrium (RA)
Tricuspid Valve (TV)
d-Transposition of Great Arteries (d-TGA)
Tetralogy of Fallot (TOF)
Left Atrium (LA)
Secundum Type Atrial Septal Defect (ASD II)
Paten Foramenobale (PFO)
Atrioventricular Valve (AVV)
Mitral Valve (MV)
Ventricular Septal Defect (VSD)
Aortic Coarctation (CoA)
Patent Ductus Arteriosus (PDA)
Great Arteries (GA’s)
Trancus Arteriosus (TA)
Double Outlet RV (DORV)
Double Outlet LV (DOLV)
Pulmonary Valvalstenosis (PAVS)
Pulmonary-to-Systemic Blood Flow Ratio (Qp/Qs)
LV Volume Overloading (LVVO)
Spontaneous Vaginal Delivery (SVD)
Alprostadil (PGE1)
Blalock-Taussig Shunt (BT-Shunt)
Total Cavopulmonary Anastomosis (TCPC)
Introduction
Definition
TVAtr is a congenital absence or agenesis of the TV. In its essence, it is a form of a hypoplastic Right Heart defect [1]. The first report, was published in 1817 by Kreysig [2].
Epidemiology
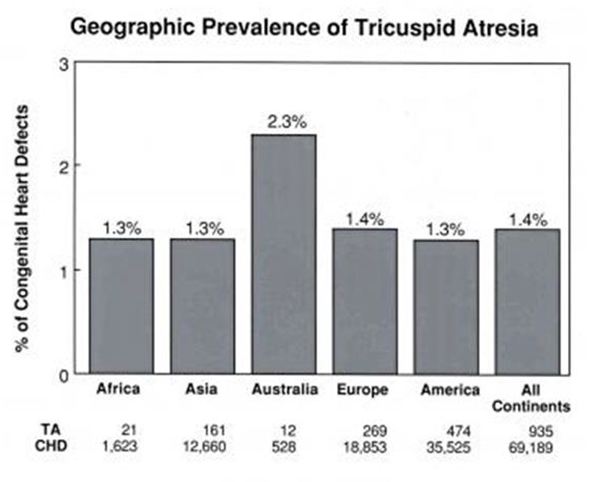
TVAtr, is described as the third most common cyanotic Congenital Heart Defect (CHD). In our series of 372 fetal scans, was the second most common, after Tetralogy of Fallot (ToF) [3]. The other 2 frequently observed were: d-TGA and DORV. Worldwide prevalence is reported between 1-2.5% of all CHD [4] (Figure 1).
Anatomical Features
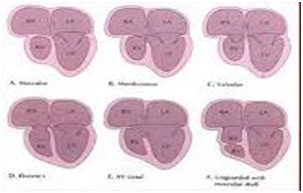
Most common histological type of TVAtr is muscular, found in 89%. Characterized by a localized fibrous thickening in the site of the TV [5] (Figure 2). Rare types are: membranous, (6.6%), in which the atrioventricular part of the membranous septum forming the floor of the Right Atrium (RA) is atretic at TV. This type is associated with absent PAV leaflets. In 2.6% of cases, an Ebstein type, combining the essence of both defects. Approximately 1% the valval cusps are minute and fused. In the atrioventricular canal type, (0.2%) an atretic leaflet of the common Atrioventricular Valve (AVV), guards the inflow to the RV [6]. In the unguarded type (0.6%), the atrioventricular junction is unguarded, but the inlet component of the RV is separated from its outlet by a muscular shelf [7] (Figure 2). Atretic TV obligatory leads all systemic venous return to progress to the Left Atrium (LA) by an intra atrial communication that in best cases is an unrestrictive secundum type Atrial Septal Defect (ASD II) and in worst a restrictive Paten Foramen Obale (PFO). Total venous return enters the LV through the Mitral Valve (MV). As disease progresses, LV dilates due to volume overload and finally, hypertrophies. This will lead to MVR+. Its severity, will correlate with the LVVO and any delay in surgical repair. An obligatory-in the classic form-VSD is present allowing the blood to enter the hypoplastic RV that size and function very, regarded to the relationship of the GA’s [8]. The VSD may be: cono-ventricular, perimembranus, cono-septal malalignment, muscular or atrioventricular canal type [9]. From these the muscular is most frequent and can restrict by time. Subpulmonary stenosis in patients with normally related GA’s and simulate sub-aortic obstruction in patients with transposition of the GA’s can be found [9]. GA’s relationship is variable and forms the basis of classification [10]. Aorta is either normal or slightly larger. Obstruction to the pulmonary outflow tract is frequent [10].
In 30% of patients, various associated cardiac defects are present, with aortic Coarctation (CoA) and persistent left superior vena cava, most common. Others are:
• Interrupted aortic arch with duct dependent Circulation.
• Absent PAV
• Aneurysm of the atrial septum
• Aorto-Pulmonary fistula
• Right aortic arch
• Anomalous Origin of the Coronary Arteries from the Pulmonary Artery (ALCAPA)
• Anomalous origin of the left and or right subclavian artery
• Common atrium
• Cor triatriatum dexter type
• Coronary sinus atrial septal defect
• Double aortic arch
• Hemi and persistent truncus arteriosus [11]
• Hypoplastic ascending aorta and/or aortic atresia primum ASD
• Uhl’s heart
• Patent Ductus Arteriosus (PDA)
• Total anomalous pulmonary venous drainage
• Tubular hypoplasia of the aortic arch
• Aortic valve stenosis
• Isomerism atrial appendages syndromes
• Anomalous drainage of coronary sinus into LA [12]
Associated extra cardiac congenital malformations are reported in 22%. Most common: chromosomal anomalies with frequent VACTERL association, unilateral Renal Agenesis, Hypospadias, Hydrothorax, Megacystis and agenesis of the Ductus Venosus [13].
Classification of the Defect
Many classifications exist. The most commonly used is based on the interrelations of the GA’s and the amount of Pav Stenosis (PAVS) that may exist [10].
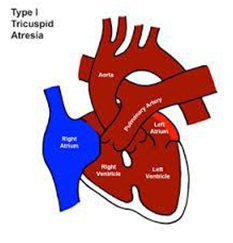
• Type I normally related GA’s (~ 70%) (Figure 3)
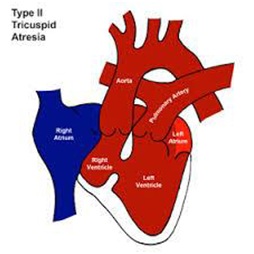
• Type II d-Transposition of the GA’s (~29%) (Figure 4)
• Type III GA’s positional abnormalities other than D-transposition: a. Subtype-1: L-transposition, b. Subtype-2: DORV, c. Subtype-3: DOLV, d. Subtype-4: D-malposition of GA’s, e. Subtype-5: L-malposition of GA’s
• Type IV TA [10,11]
• Subgroup a: With PAV atresia
• Subgroup b: With PAVS or Hypoplasia
• Subgroup c: Without PAVS
Further, the status of VSD-patent or not-and the presence of other associated malformations are described.
This unified classification [10] includes all types described by previous classifications by Kuhne, Edwards and Burchell and by Keith, Rowe and Vlad [14].
Pathophysiology
As TvAtr exists, in fetal, systemic venous blood return is forced across the PFO into the left heart. The lowered PaO2 to the brain and heart and elevated PaO2 to the lungs do not seem to produce clinical postnatal abnormalities [10]. In patients with type I-TVAtr. and associated PAV atresia types Ia and IIa, their pulmonary blood flow is supplied entirely through the PDA. PDA, delivers 8-10% of combined ventricular output compared with 66% of combined ventricular output in a normally developed fetus. Acute angulation of the PDA occurs, because of reversed direction of DA flow. These 2 factors, make the PDA less responsive to postnatal stimuli than usual.
In a fetus with type I anatomy and a small or absent VSD, almost all LV output is ejected into the aorta and transported to the placenta. Therefore, the aortic isthmus carries a larger-than-normal cardiac output; this is thought to be the reason of rare CoA in these patients.
Fetuses with type II have increased portion of the blood entering by DA into the descending aorta. This results minimal flow across the aortic isthmus. Consequence to this, leads to frequent seen CoA [15].
Postnatally, the obligatory PFO/ASDII causes a mixing of systemic and pulmonary venous returns, in the LA and passes into the LV [9,10,15]. This flow pattern occurs in all types of TVAtr. except of type III and subtypes 1 and 5. In these exceptions, the atretic TV is left sided due to ventricular inversion; therefore, the pathophysiology is that of MV atresia with consequent left-to-right shunting of pulmonary venous return [9,15].
In patients with normally related GA’s, type I and a VSD, shunting through the VSD permits perfusion of the lungs. In the VSD is absence, pulmonary blood flow is derived by a PDA or aorto-pulmonary collateral vessels [9,10,15].
In patients with D-TGA’s, type II, the lungs receive blood flow from the LV. The aorta receives blood from the LV via the VSD and RV [9,10,15]. In other types of TVAtr, the routes of aortic and pulmonary artery flow depend on the size of the VSD and associated cardiac defects. Additional conditions that affect the clinical presentation and treatment plan are:
Arterial desaturation
An amount of systemic desaturation is present in all patients, due to obligatory mixture of systemic, coronary, and pulmonary venous returns, in the LA. The degree of arterial desaturation depends on the amount of pulmonary blood-flow [9,15]. The arterial oxygen saturation has a curvilinear relationship, with a pulmonary-to-systemic blood flow ratio (Qp/Qs) that reflects the pulmonary blood flow. Qp/Qs ratio of 1.5-2.5 results in adequate oxygen saturation. Higher Qp does not significantly increase saturation and produces LVVO.
Pulmonary blood flow
Clinically, patients depend mainly on the quantity of Qp [9,16]. Neonates with markedly decreased Qp are likely to present early with severe Cyanosis, Hypoxemia, Acidosis. When Qp is increased, the neonates may appear mild cyanotic but show signs of heart failure later. Patients with pulmonary oligemia generally have type I disease. Those with pulmonary plethora usually have type II and rarely, type I c.
In patients with a type I defect, the obstruction can be valvar, sub valvar or frequently, at the VSD level. In patients with a type II defect, the obstruction is valvar or sub valvar. In patients with a type I defect, with large VSD, without PAVS, the Qp is inversely proportional to the pulmonary-to-systemic vascular resistance ratio. If a PDA or a surgical systemic-to-pulmonary artery shunt has performed, Qp, is proportional to the size of the natural or surgical shunt.
LV Volume Overloading (LVVO)
LV ejects the entire systemic, coronary, and pulmonary outputs. Therefore, LVVO is present in all patients [6,16]. The degree of LVVO increases further if mild or absent pulmonary outflow obstruction is noted or if systemic-to-pulmonary artery shunting was performed. Maintaining normal LV function is essential for a successful Fontan operation. LV function tends to decrease with increasing age, increasing Qp/Qs, and arterial desaturation [17,18].
Obstruction of the interatrial communication
PFO/ASD II, is essential for survival. As entire systemic venous blood must pass through, any obstruction if present can be clinically significant [17]. PFO/ASD II obstruction, is present when the mean pressure difference between RA-LA is > 5mm Hg [19] and/or the diameter of the defect is < 5mm [20].
Embryology
The AVV’S develop shortly after the atrioventricular cushions are activated. The anterior and posterior TV leaflets develop by undermining of a skirt of ventricular muscle tissue. The septal leaflet mostly develops from the inferior endocardial with a small contribution from the superior cushion. The process of undermining extends until the AVV junction is reached. Resorption of the muscle tissue produces normal-appearing valve leaflets and hordae tendineae. Fusion of developing valve leaflet components results in stenosis or atresia [21]. Whether a muscular type or afused valve develops depends on the embryologic stage in which aberration takes place [9,22] (Figure 2).
The embryologic, pathologic, clinical, and imaging features of TV stenosis and/or atresia are similar. This leads rare seen TV stenosis, to be calcified in this group of CHD [22,23].
Case description, treatment and 3 years follow up
The mother of the patient was referred to us for a 20th week gestation fetal echocardiography. Indications were: 1. IVF fetus following an 8 year attempt of unsuccessful spontaneous pregnancy, 2. slightly abnormal 4-chamber views were detected on the 20th week anomaly scan. No additional extra cardiac malformations were mentioned. Initially was assessed at 20+3 weeks of gestation.
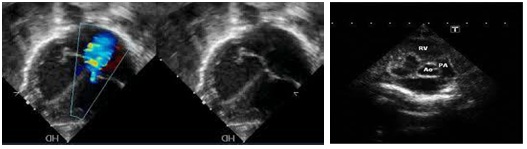
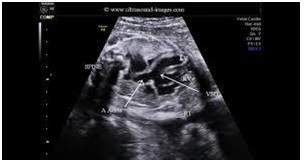
We found: Situs solitus of the abdominal organs. Left heart apex was. The cardio-thorax ratio was normal. Concordant connection between both Atrial-Ventricular and Ventricular-GA’s. Superior and Inferior vena cava were draining unrestrictedly in the RA. On the 4-chamber view the LA was dilated and an unrestrictive flow through a large intra-atrial communication in the FO with a normal directed shunt. At least 3 pulmonary veins were seen draining in LA. Discrepancy regarding the sizes of the RV and LV was noticed. There was no patency of the TV seen neither by 2D imaging nor by color mapping (Figure 5). No Doppler pulse wave was recorded through the TV. The LV was dilated and the Mv was patent. A large mid muscular VSD was seen with a left to right shunt both on 2D (Figure 6) and color mapping. The RV was smaller (Figure 6) and undeveloped, presenting with a dysplastic apex and an outlet part. The apex of the heart was created from the LV mass. The GA’s were arising normally (Figure 6). The PAV annulus was smaller in size from the Aov and their ratio was less than normal 1.1/1. The maximum velocity across the PAV was 1.8m/s. A post stenotic dilatation above the PAV was present (Figure 6). The size of the branch pulmonary arteries was normal, measuring 2.4mm. PDA with obligatory right to left shunt. The descending aorta was to the left and no evidence of CoA existed. The overall function was normal, and no pericardial infusion was present. A normal sinus rhythm was noticed with a heart rate of 141 beats per minute.
The parents were fully consultant on the findings. A detailed explanation on the possibility for progressive restriction of the PAVS to a point of critical stenosis or atresia, with a PDA dependent pulmonary circulation and the additional possibility of restriction of the PFO or VSD were explained in detail. The risk of arrhythmias or sudden intra uterus deaths of the fetus were mentioned. Detailed presentation on surgical approach in possible 3 stages towards a Fontan circulation was discussed in length [24]. The family decided to continue the pregnancy. A follow-up appointment in 4 week’s time was suggested, to assess the possible progress of the PAVS.
The next assessment revealed a similar condition except of an increase in the velocity through the PAV that was estimated at 2.2m/s and the pulmonary to aortic valve ratio that had decreased from 0.88 to 0.78. Final prenatal assessment was arranged at 33 weeks of gestation, to determine if a preterm induction of labor was needed to take place or it was safe to focus and a Spontaneous Vaginal Delivery (SVD) in a term-stage.
This took place at 33+2 weeks of gestation. The findings were unremarkable similar. The velocity across the pulmonary valve was estimated to 2.6m/s.
The baby was born by SVD at 38+4 weeks of gestation with a weight of 3.3kg. Apgar score was 8/1΄raising to 9/1΄. During her brief stay in neonatal department the pre-and-post (DA) saturation of oxygen were always measuring 93-91% respectable, failing a pulse oximetry screening test [25]. She was obligatory breast feed. The diagnosis of TVAtr. with unrestrictive ASDII and VSD, moderate hypoplasia of the RV and normally related GA’s with moderate PAVS with gradient across the PAV of 45mmHg, was established postnatally. No additional congenital malformations were found. The neonate was discharged to her home on the 5th day of her live. She was placed on a regular three months follow up in which during her first year of life her oxygen saturation dropped to 91% and the gradient across her PAV raised gradually to 65mmHg. All other parameters of her cardiac condition remained unremarkable stable. Her weight and height at her one year’s birthday were above the 50th centile and parents reported no symptoms or limitations in her activities.
During her second year of life, she was followed up every four months. Her oxygen saturation gradually dropped to 87% and the gradient across her PAV raised gradually to 80mmHg. This indicated the timing to proceed to a surgical palliation.
The patient was operated at age two years old. Her weight was then, 10.5kg’s and her pre-surgical saturation was 85%. Prior to surgery, the gradient across her PAV was 90mmHg. No MvR+ was present. Both ASDII and VSD were unrestrictive. A normal sinus rhythm was present as well as normal ventricular function.
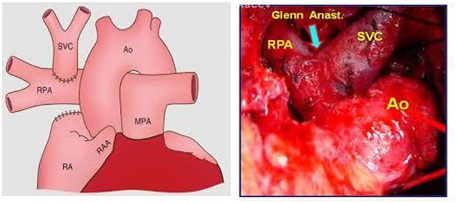
She underwent a bidirectional Glenn procedure (Figure 7), with incision of the superior vena cava, to the right pulmonary artery through a mid-sternotomy with no circulatory arrest. The child had a brief postoperative uneventful stay in the cardiac ICU. She was extubated after eight hours and transferred to a ward on the second postoperative day.
Finally, was discharged home 4 days later, on low dose aspirin (5mg/kg) [26]. Her postoperative echo-2D showed laminal flow through the SVC to the right pulmonary artery anastomosis and a normal ventricular function. No rhythm disturbances were noticed. Her saturation postoperatively raised to 94%.
Three years after her bi-directional Glenn, the patient is thriving, with a weight of 13.7kg (50th centile), a SatO2 94%, normal sinus heart rhythm with a rate of 112bpm.
A resent echo-2D, (June 2017) sowed normal ventricular function, less dilated LV than before the operation, no MvR+ and unrestrictive flow through the ASDII, VSD. Laminal low velocity flow through her SVC-Right Pulmonary Branch, Glenn Anastomosis was present. We are planning to complete the Fontal circulation in this year.
Discussion
TVAtr is a rare prenatally diagnosed CHD. Usually associated with an abnormal 4-chamber view and therefore, it must be potentially detectable during routine obstetric sonographic scans [27].
Prenatal diagnosis must emphasize on:
• No patency of TV. This can be seen by 2D and color Doppler (Figure 5)
• Cardiac axis, position, cardiothoracic ratio and situs of the viscera are normal
• Discrepancy between RV and LV is always present
• MvR+ is frequent, due to LVVO (Figure 5)
• Nearby 70% have normal related GA’s, frequently with asymmetry in the PAV/AOV ratio. Approximately 29% haved-TGA (Figure 6)
• Aortic arch in most cases is normal. The ductal arch is larger than normally, with a reversed ductal flow
• Moderate at least size VSD with a left to right shunt is frequently present (Figure 7)
• Rarely restrictive right to left flow through the FO
• The LV has a normal function and no evidence of endocardial fibro elastosis is seen in undeveloped RV [28]
When detected prenatally, a 4-week follow-up during the pregnancy is suggested to detect the final anatomy.
As extra cardiac malformations and chromosomal abnormalities is low amniocentesis is not recommended [29]. The risk of recurrence is only 1-2% in a next pregnancy [24,29]. Most are sporadic although familial cases have been reported [30].
If no prenatal data exist, postnatal diagnosis can be achieved by the failing pulse oximetry screening test [25,31]. The clinical presentation will be based on existing anatomy and physiology. If oxygen saturation is less than 70-75%, an intervention to improve pulmonary oligemia must be considered. Electrocardiogram is diagnostic, revealing RA dilatation, abnormal superiorly oriented QRS axis, LVH, and decreased RV forces. RA dilatation, with tall and peaked P waves (≥ 2.5mm) in lead D-II and right chest leads are present in 75% of patients. The so called P-tricuspidale with a double peak, spike and dome configuration may be present. Abnormal vector is present in 80% of patients with TVAtr, type I but only in 50% of patients with type II or III [32]. Echo-2D will establish the diagnosis and verify all the pathology, in most of the cases. Reveals a small RV and an enlarged RA, LA, and LV. In commonest muscular type, a dense band of echoes is observed in the TV location. The anterior leaflet of the detectable AVV is attached to the left side of the interatrial septum. These features are best demonstrated in the apical and subcostal 4-chamber views. Imaging of ASD and VSD are essential. CoA, more frequent in patients with type II, will be showed in the suprasternal view [33].
Cardiac catheterization that was so commonly used, in the era of sophisticated Echo-2D and cardiac MRI is nowadays, limited. A few cases in which specific hemodynamic data are required, verification of some of the Choussat criteria for planning surgical repair or a combination of diagnostic and interventional procedures when needed, will enter the Cath Lab [24]. An existing CoA can be addressed initially by an interventional procedure [24,34-36]. Finally, an amount of interventional procedures can be used following the surgical repair to address residual conditions.
Medicine Treatment
Neonates with severe cyanosis, pulmonary oligemia and PDA-dependent pulmonary flow can benefit by intravenous administration of alprostadil (PGE1). PGE1 is effective only in the neonatal period. A more permanent solution involving a modified or classical Blalock-Taussig Shunt (BT-Shunt) should be offered to improve cyanosis.
If congestive heart failure is present, diuretics and after load-reducing agents as Angiotensin-converting enzyme inhibitors may be administered to reduce after load and augment LV function, especially after a bidirectional Glenn procedure and a Fontan type operation. Anticoagulants and platelet-inhibiting drugs are useful in preventing thrombus formation after a Glenn or a Fontan completion.
The American Heart Association recommends that prophylaxis with antibiotics be given before any bacteremia-producing procedures are performed [37].
Surgical Treatment
The patient’s age, weight, anatomic and physiologic status determines the types of surgery recommended. The overall objective is to achieve a Fontan circulation by a total cavopulmonary connection [24].
In neonates and young infants with pulmonary oligemia, as a first step, a modified BT-shunt is undertaken to improve oxygenation.
In patients aged 6 months to 2 years, a bidirectional Glenn procedure (Figure 7) is the goal standard approach. For patients older than 2 years, total cavopulmonary connection may be performed, but most authorities suggest staging by using an initial bidirectional Glenn operation followed by Fontan completion after 6-12 months, or even after the age of 4 years old [24].
During bidirectional Glenn surgery, any narrowing of the pulmonary artery should be repaired. Other additional existing lesions such as sub-aortic obstruction and MvR+ should also be addressed [24,25,33]. Before Fontan completion, especially in delayed presenting or poorly followed up cases, some suggest cardiac catheterization to be undertaken. This approach is widely abandon as MRI studies become more frequently used. A cardiac catheterization or an MRI study need to investigate the possibility of existing aorto-pulmonary collaterals. If collateral vessels are present, they should be occluded with coils prior to the Fontan completion [24].
Most surgeons currently prefer extra-cardiac conduit diversion of inferior vena cava blood into the right pulmonary artery. To address the growth issue related to extra-cardiac Fontan surgery, some surgeons use autologous or bovine pericardial roll grafts [24,38].
In patients with D-TGA’s, early banding of the PA, relief of possible CoA, and bypassing using a Daimus-Kaye-Stansel procedure and/or resecting if technically possible of the sub-aortic obstruction should be incorporated into the management plan [24,26,38].
Following the Fontan procedure, immediate, mid and long-term complications that can occur are: persistent pleural effusions, neurologic complications such as strokes or brain abscesses, arrhythmias, thromboembolic events, obstruction at the anastomotic sites or in the pulmonary artery, residual shunts, and/or systemic venous congestion presenting as protein-losing enteropathy and rarely plastic bronchitis. All these need to be aggressively treated to sustain a New York Heart Association stage I to II and improve quality and life expectancy [24].
In patients with “Failing Fontan” after excluding/addressing obstructions, residual shunts and arrhythmias, apart from conventional treatment, consideration for resynchronization by pacing the RA and LV and conversion of atrioventricular Fontan’s to Total Cavopulmonary Anastomosis (TCPC). Cardiac transplantation should be considered as the last step of treatment [24,39,40].
Life Style Implications
In most patients, no dietary restrictions are necessary. In infants with failure to thrive, a high-calorie formula delivering 24 to 30 cal/30gr may be needed to ensure adequate weight gain. Lower amounts of diluent water combined with glucose polymers (Polycose) and medium-chain triglycerides may be used. Finally, in post-operative TCPC, long standing plural effusions or chylothorax, a diet rich in medium-chain triglycerides may be useful [24].
No specific exercise restrictions are recommended. Participation in school physical education must be patient tailored.
Changes in lifestyle, to avoid highly exertional activities, such as competitive sports or work that requires substantial physical activity, are necessary.
Traveling or living at altitudes higher than 1850m from the sea level should be undertaken carefully and with medical assessment [41].
Due to significant and important hemodynamic and hematologic changes occurring during pregnancy, this is restricted -under conditions-to the very best repaired patients. Maternal mortality rate is 14% and miscarriages occur in 54% of patients. High incidence of prematurity, small-for-gestational-age and perinatal mortality have been reported. Limited data from pregnancies after Fontan completion, suggest that acceptable outcomes may result if the LV function is excellent and the functional NYHA class is I or II at the time of conception [42].
Prognosis
Prognosis in untreated TVAtr is poor. Early identification, rapid and safe transport to a pediatric cardiology center, noninvasive diagnosis, availability of PGE1 to keep PDA, and recent advances in anesthesia and surgical techniques have improved the prognosis of these babies. Complications associated with the classic Fontan are decreased with the widespread use of staged TCPC [24,32].
Conclusion
TVAtr is a complex cyanotic critical CHD requiring multiple medical, intervention and surgical treatment. Prenatal diagnosis is available alternating early morbidity and mortality but also offers to parent the alternative of an elective abortion. A detailed explanation of the cardiac defect and treatment required should be given to the parents at the time of diagnosis and repeated as needed. Further, pulse oximetry screening after the 3rd to 4th day postnatal can detect this CHD [25].
Acknowledgment
The authors want to thank Dr’s Aynur Mustafayeva of Merkezi Klinika and Tarana Maharammova of XMS Clinic for their efforts regarding this patient.
Conflict of Interest
None of the authors have any conflict of interest regarding this presented case