Introduction
Bone grafting in foot and ankle surgery for fractures, osteotomies, and arthrodesis is a common practice [1]. Due to concerns over autogenous donor site morbidity [2,3] graft substitutes have been developed. In foot and ankle surgery, two main autograft alternatives have been put forward as alternatives for high risk procedures, recombinant human Bone Morphogenetic Protein (BMP) and allograft viable Mesenchymal Stem Cells (MSC). BMPs are powerful osteoinductive graft materials, and have demonstrated clinical effectiveness in nonunions, segmental defects, and long bone fractures [4-7]. Evaluation of its application (off-label) in foot and ankle surgery has generally been favorable [8-12]. On the other hand, MSCs have also demonstrated effectiveness in surgery [13]. Commercially available MSCs approved for the use in foot and ankle arthrodesis provide osteogenic, osteoconductive, and osteoinductive properties, and have been recommended in various applications [14-16].
A novel processing method has provided a way to obtain Allogeneic Morphogenetic Protein (AMP) from allograft and its native bone marrow. This process has been purported to allow for higher concentrations of growth factors (such as BMP-2, BMP-7, Transforming Growth Factor (TGF)-β1) to bond to the scaffold and be delivered to the operative site [17]. AMP (OsteoAMP, Bioventus LLC, Durham, NC, USA) is available in compressible sponge, putty, and granule formats, and can be combined with Bone Marrow Aspirate (BMA). Also, it has been evaluated in several clinical trials in the spine. Fusion rates in spinal surgery were not affected by graft variability, and specifically looked at donor age, donor sex, age of graft from harvest to implantation, and aseptic or terminal irradiation [18], indicating predictable quality of the product. It was also evaluated in cervical spine surgery, and demonstrated high fusion rates [19]. A study of 321 patients undergoing various spine fusion operations was undertaken to compare AMP to BMP. The 226 patients in the AMP arm compared very well to the 95 patients in the BMP arm in terms of fusion rate, time to fusion, and complications [20].
AMP is approved for use in situations where an autograft is appropriate and can be used for repair, replacement or reconstruction of skeletal defects, and has on-label use in the foot and ankle. The various growth factors provide osteoinduction, the various formats provide osteoconduction, and when combined with BMA provides autogenous osteogenic cells [21], thus replicating all three pillars of bone healing. This study was undertaken to evaluate initial safety and efficacy of AMP in foot and ankle surgery. The hypothesis was that AMP will have outcomes in foot and ankle surgery comparable to other products with no unexpected complications. The primary objective was to evaluate safety of its use in foot and ankle surgery, with the secondary objective of time and rate of union.
Patients and Methods
Institutional review board approval (15-50E) was obtained for the present study. A retrospective chart and radiographic review was performed on the first ten patients that underwent foot and ankle utilizing AMP (OsteoAMP) from November 2014 to February 2015. Patients were identified by review of cases utilizing AMP. Inclusion criteria included all patients that underwent surgical intervention and utilized AMP. Exclusion criteria included incomplete patient information, or lack of use of AMP. Indication for use of AMP was surgeon dependent, but was selected in high risk patients. This included revision surgery, smoking, medical co-morbidities, advanced age, and obesity. Surgery was performed using standard surgical technique dependent on the specific surgical indication. All surgeries were performed by one of the two authors, fellowship trained foot and ankle surgeons. Each surgeon contributed 5 patients to the current study.
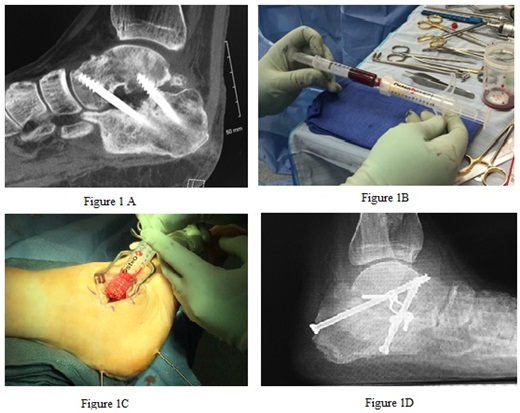
Although surgical indications, approach, and fixation were different for the various cases, certain standard techniques were utilized. In all revision operations, the same incision was reused and all impeding hardware was removed. All cases focused on bone fusion, whether primary arthrodesis, revision of arthrodesis nonunion, or fracture nonunion. In all cases, the proposed fusion site was exposed, and any remaining articular surface was removed past subchondral bone. For nonunion, this included debridement of fibrotic material to raw, bleeding bone. The area was then fenestrated with small drill or pin. Once the arthrodesis site was prepared, it was packed with AMP and any other grafting material used. Fixation was specific to the anatomic site, but in all cases was meant to provide compression and stability. Forefoot cases were allowed to weight bear in a protective boot at 2 weeks, while all midfoot, hindfoot, and ankle cases were restricted to a non weight bearing splint and then fiberglass cast for 6-8 weeks (Figure 1).
Medical chart review recorded age, gender, ethnicity, past medical history, social history, indication, time to weight bearing, and complications. Radiographic review was undertaken to determine time to radiographic fusion. This was defined as radiographic evidence of bridging bone, with lack of motion or hardware complications. This was performed by the authors. Since no comparisons are to be made, only simple statistical analysis was performed. Patient demographics will be presented as mean with standard deviation and ranges of continuous variables such as age, time to weight bearing, and time to radiographic union. In order for the surgery to be classified as union, bridging bone needed to be appreciated on plain films, and the patient had to be clinically fused with no further swelling or pain. This was assessed by the operating surgeon. Categorical variables such as gender, ethnicity, past medical history and social history, and complications will be represented as percentages of the group.
Results
The study population included 5 females (50%), and 5 males (50%), with an average age of 57.3 ± 13.3 years (range: 29-80 years). All patients were Caucasian. Patients had a variety of medical co-morbidities, including Diabetes Mellitus (DM), chronic kidney disease, and a variety of mental disorders (Table 1). Social risk factors were recorded, and demonstrated that 4 patients (40%) never used tobacco and 5 patients (50%) did not use any alcohol. Of the patients that smoked, only 1 was an active smoker, and the others had quit an average of 18.0 ± 8.8 yrs (range: 8-32 years) prior to the studied operation. The one patient that was an active smoker also drank 15 drinks per week, and used marijuana daily, and was the only patient that related illegal drug use. Patients were followed an average of 167.6 ± 21.8 days (range: 125 - 188 days) from surgery.
Mental disorder includes one or more of the following: Attention deficit disorder, Obsessive compulsive disorder, Anxiety, Depression
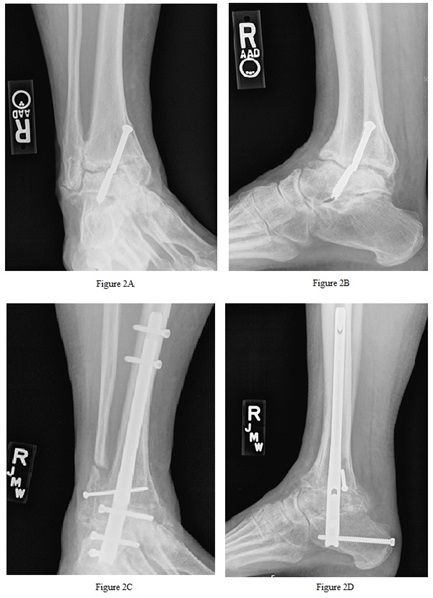
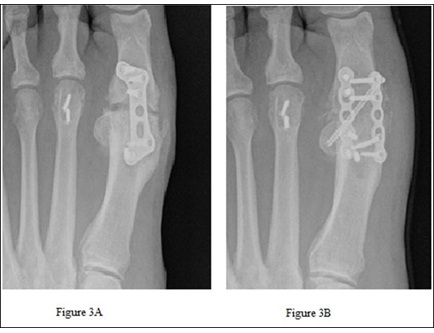
The patients underwent a variety of surgical treatments. Nonunion of previous arthrodesis or of fracture or revision of failed prior surgery was the indication for 5 patients (50%) (Figures 2 and 3). A total of 23 fusion sites were represented in the study population. Adjunctive procedures were performed in 7 patients (70%). Autogenous grafting was utilized in 3 patients (30%) and BMA was used in 5 patients (50%). A detailed list of surgical variables is listed in table 2.
Ultimately, 22 out of 23 fusion sites (95.7%) in 9 patients (90%) went on to solid arthrodesis. The average time to weight bearing was 59.9 ± 20.6 days (range: 14 - 78 days). Average time to radiographic healing was 78.5 ± 42.4 days (range: 42 - 187 days). Overall, 2 patients (20%) experienced complications. This included 1 wound dehiscence that was treated with local wound care and oral antibiotics, and 1 nonunion of the medial malleolus. The nonunion was stable and the patient has not undergone revision surgery.
Discussion
Bone grafting procedures are frequently performed in orthopedic surgery, with more than 500,000 bone grafting procedures performed each year in the United States alone [22]. Historically, autogenous bone graft has been considered the “gold standard”, and typically has been discussed as graft taken from the iliac crest. This has been described in spine surgery [23,24] and also specifically evaluated in foot and ankle surgery [25-27]. However, because of complications associated with the harvest of the autogenous graft, alternatives to the iliac crest have proliferated [28-30]. This has led to development of over 200 different commercially available bone graft substitutes, some of which can be very expensive and most with little or no high level evidence to support their use [31]. Research continues to find the ideal autogenous bone graft alternative. Ideally, this will be something that is effective, safe, readily available, non-morbid and relatively inexpensive.
An article by Roh et al., in 2013 was published comparing recombinant human BMP (rhBMP-2, Infuse, Medtronic Inc., Fridley, MN, USA) to AMP (OsteoAMP) in lumbar interbody fusion procedures [20]. This was a multicenter study comparing the fusion rate of the two products, as well as the cost associated with them. A total of 321 consecutive patients were evaluated over 5 years, 95 in the BMP group and 226 in the AMP group. The study reported universally favorable findings of AMP versus BMP, including time to fusion, percentage fusion, and complications. In addition, the AMP group also reported approximately 80% lower costs as well (about $650 for AMP vs $2500 for BMP). Several concerns are raised about the article. Statistical analysis of the findings between the groups was not consistently reported, and there was a potential commercial bias [32]. Even with these limitations, the results were impressive and lead to the current investigation.
The AMP used in this investigation not only has favorable clinical studies [18-20], but internal data and analysis also compares favorably to competing products. It shows high levels of BMP-2, BMP-7, and TGF-β1, and others in the sponge product [17]. In addition to the clinical and laboratory data, the product is also practical and versatile in the surgical setting and has excellent handling abilities. It is available in a compressible sponge, and has higher concentrations of BMP-2 than another commercially available compressible sponge product (Bacterin Osteosponge; Bacterin International Holdings, Inc., Belgrade, MT, USA) [17]. The authors in the present study utilized the putty or the granule forms and mixed with BMA in 50% of the cases. Bone marrow aspirate can quickly, easily, and safely be harvested from lower extremity sites, and although there are fewer colony-forming units in lower extremity sites compared to the iliac crest, only a minimal concentration of cells may be needed to achieve clinical fusion [33-35].
High risk foot and ankle fusions are often augmented with some biologic adjunct. Two options that have been recommended are recombinant human BMP and MSC. After extensive study in long bone fractures, segmental defects, and nonunions, recombinant human BMP was explicitly studied in the foot and ankle. A study published in 2009 by Schuberth et al., performed a multicenter evaluation of recombinant human BMP in foot and ankle surgery. Overall, out of 38 procedures in 35 patients, the incidence of successful healing was 84.2% (32 procedures), and multiple statistical analyses teased out predictors of success [8]. Another study in 2009 evaluated 69 patients, but broke the results down to 112 fusion sites augmented with recombinant human BMP. The authors reported a 96% fusion rate (108 sites), and this was confirmed with CT scan. The patient population consisted of 64% smokers, 19% diabetic patients, 68% high energy trauma, and 22% Avascular Necrosis (AVN) of the talus. In addition to the excellent fusion rate, low complication rates were reported as well [11]. DeVries et al., in contrast reported a 69.6% (16 of 23 patients) rate of arthrodesis for revision nonunion cases treated with intramedullary tibiotalocalcaneal nail. No clear benefit was demonstrated with the addition of recombinant human BMP, although again no specific adverse effects of the BMP were seen [12].
Similarly, evaluation of MSCs has been undertaken in the foot and ankle. Rush et al., published several studies looking into the use of MSCs, including a retrospective review of 23 patients in 2009. Here the authors found an overall union rate of 91.3% (21 patients), and observed new bone formation in all patients at the level of implantation. No graft specific complications were found [14]. Scott and Hyer also evaluated this graft in 20 patients in 2013. All patients were undergoing primary arthrodesis, but were classified as “high-risk” based on smoking status, high Body Mass Index (BMI), or presences of diabetes. The authors presented a 90% (18 patients) union rate, with 2 patients resulting in nonunion that required additional surgery. Again, no graft specific complications were noted [15].
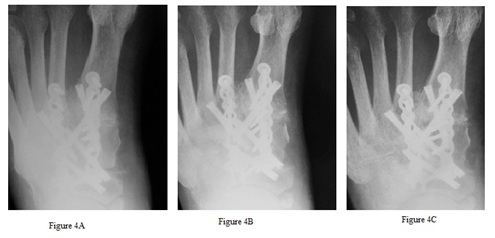
The current study demonstrates that AMP compares very well to both BMP and MSC. There was successful arthrodesis in 90% (9 out of 10) patients, and this included 95.7% (22 out of 23) total arthrodesis sites. The graft also demonstrated new bone formation in these patients (Figure 4). This included “high-risk” patients including revision surgery, diabetics, advanced age, and smokers. AMP has some notable advantages over these other adjuncts. Recombinant human BMP (Infuse) is indicated for spinal fusion and open tibia fractures, and is not specifically intended for use in the foot and ankle, and has been reported to have some adverse events in the spine and an increase in cancer incidence of 1.8-3% [36]. It also has poor handling characteristics and does not provide any space-filling properties. The viable cells in MSC are a benefit, but also have drawbacks. The product must be stored in a freezer at -70˚C to -80˚C and has a shelf life of 3 months. Careful thawing of the product has to be undertaken to ensure cell viability, and must be used within 2 hours of thawing [16]. The AMP product provides high levels of BMP-2 as well as a variety of other growth factors. It provides a scaffold and space-filling properties, and when mixed with autogenous BMA can provide all three properties of bone healing. It is freeze dried and can be stored at room temperature for 5 years. Finally, the AMP used in this study (OsteoAMP) is substantially less expensive than other products at the authors institution. A package of 5 cc granules is priced at $1755.00, compared to medium Infuse® priced at $4893.00 and 5 cc of Trinity Evolution (Orthofix, Verona, Italy) priced at $2259.00. Although a cost comparative analysis is beyond the scope of this paper, cost advantages were cited in spine literature [20].
The single incidence of nonunion was present in a patient that smoked tobacco and marijuana daily, was a heavy drinker (15 drinks/week), and was non-compliant with his non-weight bearing status immediately after surgery. He had presented 3 months after displaced trimalleolar fracture with nonunion at all 3 fracture sites, and stated that his pain had been well controlled throughout, likely due to his daily marijuana use, which he admitted to increasing after the injury. This also was likely a determining factor in his recovery as he stated he had very little pain and did not require pain medications after surgery, despite early weight bearing and having an obvious medial malleolar nonunion. And even with this, he did go onto a solid union of his fibular nonunion.
This retrospective study was intended to be an early analysis of the first patients in which the AMP product was utilized. This was done purposely because of the limited amounts of available literature describing this product, and an uncertainty in the expected outcomes in foot and ankle surgery. There are several important limitations that were accepted at the outset of this report. There is no comparative group, there is a small sample size, and the follow up is short. Without a control or comparative group it is impossible to determine how many of these patients would have healed simply with the revision operation. There is no patient functional data or high level statistical analysis. In addition, several patients had autogenous grafting used in conjunction to the AMP which could have influenced the results. The inclusion criteria was at the total discretion of the operating surgeon as this represented the first cases in which AMP was used, and thus could introduce bias. Bias could also be introduced in the radiographic determination of union. CT scan would have been ideal, but as an early look at these patients, the cost and additional radiation was deemed unnecessary for the purposes of the paper. The aim of the report was to explore the use of a novel allograft bone graft alternative, and establish whether this seemed to be safe and effective for these high risk operations. Based on these early findings, a more robust clinical trial is planned, and needs to be undertaken to delineate this products place in the surgeons’ armamentarium.
In conclusion, AMP is found to have similar outcomes as would be expected for other studied bone graft materials. It is difficult to draw firm conclusions based on this early evaluation due to the stated limitations. However, no graft specific adverse reactions were identified. There are several practical advantages to the current product, and further study was deemed warranted. Based on these findings, the authors are collecting further data to address several of this limitations. Advanced biologics such as the one explored in this work continue to show great promise and utility in foot and ankle surgery, especially in the face of higher risk populations and procedures.
Financial Disclosure
Dr. DeVries is a consultant with Bioventus, owner of OsteoAMP
Figures
Figure 1: A Shows a CT scan revealing nonunion at subtalar joint with hardware failure. B is preparation of 5 mL of allogeneic bone morphogenetic protein granules being mixed with 6 mL of bone marrow aspirate. C shows delivery of grafting materials into the nonunion site. D is a weight bearing lateral plain radiograph at 3 months post-operatively showing solid arthodesis with alternative hardware placement.
Figure 2: A (mortise) and B (lateral) are pre-operative images of patient 3 demonstrating ankle nonunion with anterior translation, subtalar joint arthritis, broken hardware. C (mortise) and D (lateral) are post-operative images demonstrating solid union at the ankle and subtalar joints, as well as the fibula onlay site.
Figure 3: Patient 1 shows pre-operative nonunion of the 1st metatarsal phalangeal joint with broken proximal screw (A) and post-operative arthrodesis (B).
Figure 4: New bone growth is demonstrated in patient 7. AP image two weeks post-operative (A) shows open joints at the 1st tarsometatarsal and medial naviculocuneiform. AP image at 48 days post-operative (B) shows solid arthrodesis at those joints. AP image at 139 days post-operative (C) demonstrates new bone growth at the 1st interspace between the 1st and 2nd metatarsal base.