
Journal of Pulmonary Medicine & Respiratory Research Category: Medical
Type: Research Article
Evaluation of Respiratory Virus Pathogen from Patients Presenting with Influenza like Illness at Various Hospitals in Delhi, India
*Corresponding Author(s):
Madhu KhannaDepartment Of Respiratory Virology, Vallabhbhai Patel Chest Institute, University Of Delhi, New Delhi, India
Tel:+91-11-27402432,
Email:madhukhanna@hotmail.com
Received Date: Apr 29, 2018
Accepted Date: May 20, 2019
Published Date: May 27, 2019
Abstract
Background
Respiratory viruses have been widely circulating in human populations and its variants have caused, and continue to cause, substantial morbidity and mortality worldwide.
Objective
The objective of this study was to compare the presence of various circulating respiratory virus among the population in Delhi region.
Materials and Method
The epidemiology and burden of human respiratory viruses were examined in a cohort of 106 patients from Delhi, India, by using real-time quantitative reverse transcription polymerase chain reaction.
Results
Of the 106 screened samples tested,35.84% patients were found positive for influenza A virus, 19.81% for Human Metapneumovirus (hMPV),5.66% for Rhinovirus (HRV), 3.77%for Parainfluenza (PIV) type 4 and Human Enterovirus (HEV), 2.83% for Human Coronavirus (HCoV) OC43, while 0.94% for influenza B, coronavirus NL63 and 229E, Parainfluenza type-2 and 3 and Bocavirus. The analysis revealed that Parainfluenza (PIV-1, 2 and 3), Respiratory Syncytial Virus (RSV) A and B, and Human Bocavirus (HBoV) are not commonly circulating among general population.
Conclusion
The metapneumovirus and influenza A virus are important respiratory pathogens in patients. Apart from these HRV and HEV embark to the clinical significance in child care. Influenza and HRV caused the highest-impact illnesses. The current study depicts the burden of respiratory viruses in current population.
Respiratory viruses have been widely circulating in human populations and its variants have caused, and continue to cause, substantial morbidity and mortality worldwide.
Objective
The objective of this study was to compare the presence of various circulating respiratory virus among the population in Delhi region.
Materials and Method
The epidemiology and burden of human respiratory viruses were examined in a cohort of 106 patients from Delhi, India, by using real-time quantitative reverse transcription polymerase chain reaction.
Results
Of the 106 screened samples tested,35.84% patients were found positive for influenza A virus, 19.81% for Human Metapneumovirus (hMPV),5.66% for Rhinovirus (HRV), 3.77%for Parainfluenza (PIV) type 4 and Human Enterovirus (HEV), 2.83% for Human Coronavirus (HCoV) OC43, while 0.94% for influenza B, coronavirus NL63 and 229E, Parainfluenza type-2 and 3 and Bocavirus. The analysis revealed that Parainfluenza (PIV-1, 2 and 3), Respiratory Syncytial Virus (RSV) A and B, and Human Bocavirus (HBoV) are not commonly circulating among general population.
Conclusion
The metapneumovirus and influenza A virus are important respiratory pathogens in patients. Apart from these HRV and HEV embark to the clinical significance in child care. Influenza and HRV caused the highest-impact illnesses. The current study depicts the burden of respiratory viruses in current population.
Keywords
Disease burden;Epidemiology;Human metapneumovirus; Influenza virus; Parainfluenza virus;Respiratory virus;Rhinovirus;RT-PCR
INTRODUCTION
Acute Respiratory Infections (ARI) are a major global public health problem and widely circulating in human population. Despite progress made in the 20th century with the introduction of antibiotics, vaccines and antivirals, there are no specific interventions for most respiratory infections of viral origin. Although, the recent years have witnessed a tremendous development in the management of respiratory viruses including influenza and other respiratory viral infections, the world still suffers from these viral infections every year leading to an increase in morbidity and mortality[1-4].
These infections continue to cause frequent morbidity, and sometimes cause severe outcomes including death, especially in developing countries [5-6]. ARI can be the leading cause of morbidity in young children, and accounted for one-fifth of all deaths in children less than five years of age. Of those mortalities, 70% occurs in Africa and Southeast Asia [7]. Approximately one-third of children develop Lower Respiratory Tract Infections (LRTI) in the first year of life [8].
The major causes of Acute Respiratory Distress Syndrome (ARDS) in children and adults are influenza A and B viruses, Parainfluenza Virus (PIV) type 1-3, Respiratory Syncytial Virus (RSV), adenovirus, and rhinovirus. Other viruses such as Coronavirus (CoV), human bocavirus, human enterovirus, PIV-4, the newly discovered parvovirus types 4 and 5, and mimivirus also infect the respiratory tract albeit at a much lower frequency [9-11]. Rhinoviruses and CoVs were identified as human pathogens in the 1960s [12], but they have been largely ignored by the medical community because their clinical impact was considered to be minor. The rhinoviruses and CoVs, once thought to cause only common cold, can cause LRTI and ARDS and can be fatal in few cases. Indeed, all of the viruses mentioned above have overlapping clinical presentations and cause both upper respiratory tract infection (URTI) and LRTI, and cannot be distinguished by attending physicians unless a laboratory diagnosis is done for the causative agent [11].
Influenza virus is known to cause annual epidemics in temperate climates that are characterized by a sudden increase in febrile respiratory illness; the epidemic period is generally 3 to 8 weeks [11,12]. Although the four serotypes of PIV that infect human has the similar mode of transmission and pathogenesis, they don’t undergo antigenic shift and drift [13]. PIV-1 is the major cause of acute croup in infants and young children but also causes mild URTI, pharyngitis, and tracheobronchitis in all age groups. PIV-2 is generally associated with lower infection rates than PIV-1 or PIV-3 and has been associated with mild URTI, croup in children, and, occasionally, LRTI. PIV- 3 is a major cause of severe LRTI in infants and young children, often causing croup, bronchitis, and pneumonia in children <1 year of age [11]. In older children and adults, it can cause URTI or tracheobronchitis [14].
RSV is another etiological agent causing respiratory disease in infancy and is a major cause of bronchiolitis and pneumonia in infants under 2 years of age [15]. hMPV causes both URTI and LRTI with similar symptoms ranging from mild rhinorrhea associated with common colds to severe cough, wheezing, bronchiolitis, and pneumonia [15,16].The adenovirus is pervasive, and infections are prevalent; only the serotypes 1 to 5, 7, 14, 19, and 37 infect the respiratory tract, causing a variety of mild symptoms including fever, rhinitis, pharyngitis, cough, and conjunctivitis and more severe disease including laryngitis, croup, bronchiolitis, or pneumonia, on occasion [17,18]. Most adenovirus infections occur early in life, and by age of 10 years, most children are infected with at least one serotype [19].
Human rhinoviruses are perhaps the most captivating of the conventional viruses as revealed by the recent reports of higher-than-expected infection rates in hospitalized children with acute LRTI [20-25]. Rhinoviruses were believed to cause only “the common cold” and thus were neglected by the medical community until not long ago, when their spectrum of disease expanded.
Among other respiratory viruses, the enteroviruses are ubiquitous agents found worldwide [26]. It has been estimated that enteroviruses infect 1 billion or more individuals worldwide each year. Several modes of transmission exist for these viruses, including fecal-oral, respiratory, transplacental, perinatal, and self-inoculation modes, but the majority are fecal-oral [27,28]. The highest incidence of enterovirus infection is in infants and young children [29]. The majority of enterovirus infections are asymptomatic [30]. HCoV-OC43 and HCoV-229E were identified in the mid-1960s as being a cause of mild self-limited URTI and were subsequently shown to cause about one-third of “common cold”-like illnesses in adults. Like other respiratory viruses, HCoV-OC43 and 229E are spread by large droplet infection. Overall, they account for 5 to 30% of respiratory tract infections, and outbreaks may occur in 3 to 4 years interval [31]. HCoV-OC43 and 229E has been associated with both URTI and LRTI in a variety of settings including nosocomial infections in high-risk immune-compromised children, in hospitalized elderly patients with non-influenza-virus ARD and pneumonia, and in newborns, children and hospital staff [32-34]. The recent findings by the infection control practitioners show that HCoV-229E can survive for up to 3 hours when dried on solid surfaces and for up to 6 days in saline solution at room temperature [35].
In most studies where the frequencies of several viruses were determined, HBoV was most prevalent in children <3 years of age and less common than RSV and rhinovirus. HBoV infections occur in both children and adults, but children under the age of 2 years appear to be mostly at risk of infection [35].
These infections continue to cause frequent morbidity, and sometimes cause severe outcomes including death, especially in developing countries [5-6]. ARI can be the leading cause of morbidity in young children, and accounted for one-fifth of all deaths in children less than five years of age. Of those mortalities, 70% occurs in Africa and Southeast Asia [7]. Approximately one-third of children develop Lower Respiratory Tract Infections (LRTI) in the first year of life [8].
The major causes of Acute Respiratory Distress Syndrome (ARDS) in children and adults are influenza A and B viruses, Parainfluenza Virus (PIV) type 1-3, Respiratory Syncytial Virus (RSV), adenovirus, and rhinovirus. Other viruses such as Coronavirus (CoV), human bocavirus, human enterovirus, PIV-4, the newly discovered parvovirus types 4 and 5, and mimivirus also infect the respiratory tract albeit at a much lower frequency [9-11]. Rhinoviruses and CoVs were identified as human pathogens in the 1960s [12], but they have been largely ignored by the medical community because their clinical impact was considered to be minor. The rhinoviruses and CoVs, once thought to cause only common cold, can cause LRTI and ARDS and can be fatal in few cases. Indeed, all of the viruses mentioned above have overlapping clinical presentations and cause both upper respiratory tract infection (URTI) and LRTI, and cannot be distinguished by attending physicians unless a laboratory diagnosis is done for the causative agent [11].
Influenza virus is known to cause annual epidemics in temperate climates that are characterized by a sudden increase in febrile respiratory illness; the epidemic period is generally 3 to 8 weeks [11,12]. Although the four serotypes of PIV that infect human has the similar mode of transmission and pathogenesis, they don’t undergo antigenic shift and drift [13]. PIV-1 is the major cause of acute croup in infants and young children but also causes mild URTI, pharyngitis, and tracheobronchitis in all age groups. PIV-2 is generally associated with lower infection rates than PIV-1 or PIV-3 and has been associated with mild URTI, croup in children, and, occasionally, LRTI. PIV- 3 is a major cause of severe LRTI in infants and young children, often causing croup, bronchitis, and pneumonia in children <1 year of age [11]. In older children and adults, it can cause URTI or tracheobronchitis [14].
RSV is another etiological agent causing respiratory disease in infancy and is a major cause of bronchiolitis and pneumonia in infants under 2 years of age [15]. hMPV causes both URTI and LRTI with similar symptoms ranging from mild rhinorrhea associated with common colds to severe cough, wheezing, bronchiolitis, and pneumonia [15,16].The adenovirus is pervasive, and infections are prevalent; only the serotypes 1 to 5, 7, 14, 19, and 37 infect the respiratory tract, causing a variety of mild symptoms including fever, rhinitis, pharyngitis, cough, and conjunctivitis and more severe disease including laryngitis, croup, bronchiolitis, or pneumonia, on occasion [17,18]. Most adenovirus infections occur early in life, and by age of 10 years, most children are infected with at least one serotype [19].
Human rhinoviruses are perhaps the most captivating of the conventional viruses as revealed by the recent reports of higher-than-expected infection rates in hospitalized children with acute LRTI [20-25]. Rhinoviruses were believed to cause only “the common cold” and thus were neglected by the medical community until not long ago, when their spectrum of disease expanded.
Among other respiratory viruses, the enteroviruses are ubiquitous agents found worldwide [26]. It has been estimated that enteroviruses infect 1 billion or more individuals worldwide each year. Several modes of transmission exist for these viruses, including fecal-oral, respiratory, transplacental, perinatal, and self-inoculation modes, but the majority are fecal-oral [27,28]. The highest incidence of enterovirus infection is in infants and young children [29]. The majority of enterovirus infections are asymptomatic [30]. HCoV-OC43 and HCoV-229E were identified in the mid-1960s as being a cause of mild self-limited URTI and were subsequently shown to cause about one-third of “common cold”-like illnesses in adults. Like other respiratory viruses, HCoV-OC43 and 229E are spread by large droplet infection. Overall, they account for 5 to 30% of respiratory tract infections, and outbreaks may occur in 3 to 4 years interval [31]. HCoV-OC43 and 229E has been associated with both URTI and LRTI in a variety of settings including nosocomial infections in high-risk immune-compromised children, in hospitalized elderly patients with non-influenza-virus ARD and pneumonia, and in newborns, children and hospital staff [32-34]. The recent findings by the infection control practitioners show that HCoV-229E can survive for up to 3 hours when dried on solid surfaces and for up to 6 days in saline solution at room temperature [35].
In most studies where the frequencies of several viruses were determined, HBoV was most prevalent in children <3 years of age and less common than RSV and rhinovirus. HBoV infections occur in both children and adults, but children under the age of 2 years appear to be mostly at risk of infection [35].
MATERIALS AND METHODS
Study design
We retrospectively studied all critically ill patients with Influenza Like Illness (ILI) from Military Base Hospital, Delhi; Kalawati Saran children Hospital, Delhi and V.P. Chest Institute, Delhi from September, 2012 to August, 2013. The patients with influenza-like-illness and respiratory complications presenting to the Out-Patient Department (OPD) during the study period underwent diagnostic testing for evidence of a panel of respiratory virus [36].Appropriate clinical specimens (throat swabs and nasal swabs) were collected in viral transport medium, as described previously following Centers for Disease Control and Prevention (CDC), Atlanta and WHO guidelines prior to informed consent from the patients or guardian[37-39]. All the collected clinical specimens were subjected to one-step Reverse Transcriptase-PCR (RT-PCR) for a panel of respiratory viruses, from Seeplex RV15 one-step Ace detection kit (Seegene, Korea) as per manufacturer’s instructions. Ethical clearance for conducting the study was obtained from the Institute’s Ethics Committee.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation (SD) and median with interquartile ranges. Categorical variables were presented as numbers and percentages. The Statistical graphs were made through analyzing data with Graph Pad Prism software version 5.5.
RESULT
During the stipulated study period, patients with respiratory illness were evaluated; a total of 106 patients of different age group were screened.
Of the 106 screened samples, 38 (35.84%) patients were found positive for influenza A virus followed by 21 (19.81%) for Metapnemovirus, 6 (5.66%) for Human Rhinovirus, 4 (3.77%) for Parainfluenza type 4 and Human Enterovirus, 3 (2.83%) for Human Coronavirus OC43, while one each (0.94%) for influenza B, Human Coronavirus NL63 and 229E, Parainfluenza type-2 and 3 and Human Bocavirus. While evaluating cases, it was found that Parainfluenza 1, 2 and 3, RSV A and B, and Human Bocavirus were not commonly circulating in general population.
The mean age of patients infected with influenza was 33.55+19.16followed by 9.69+13.65 for hMPV, 3.49+3.80 for HRV, 25.72+40.95 for PIV4, 0.41+ 0.58 for coronavirus OC43, 4.93+3.71 for HEV and single cases of influenza B, coronavirus NL-63 and 229E, HBoV, PIV type 2and3. In comparison, the mean age was found to be 3.83+3.48 for patient group below 18 years, followed by 43.23+16.93 for patient group above 18 years. It was observed that the most of the cases were in the range of 0-18 year age group (Figures 1 and 2).
Of the 106 screened samples, 38 (35.84%) patients were found positive for influenza A virus followed by 21 (19.81%) for Metapnemovirus, 6 (5.66%) for Human Rhinovirus, 4 (3.77%) for Parainfluenza type 4 and Human Enterovirus, 3 (2.83%) for Human Coronavirus OC43, while one each (0.94%) for influenza B, Human Coronavirus NL63 and 229E, Parainfluenza type-2 and 3 and Human Bocavirus. While evaluating cases, it was found that Parainfluenza 1, 2 and 3, RSV A and B, and Human Bocavirus were not commonly circulating in general population.
The mean age of patients infected with influenza was 33.55+19.16followed by 9.69+13.65 for hMPV, 3.49+3.80 for HRV, 25.72+40.95 for PIV4, 0.41+ 0.58 for coronavirus OC43, 4.93+3.71 for HEV and single cases of influenza B, coronavirus NL-63 and 229E, HBoV, PIV type 2and3. In comparison, the mean age was found to be 3.83+3.48 for patient group below 18 years, followed by 43.23+16.93 for patient group above 18 years. It was observed that the most of the cases were in the range of 0-18 year age group (Figures 1 and 2).
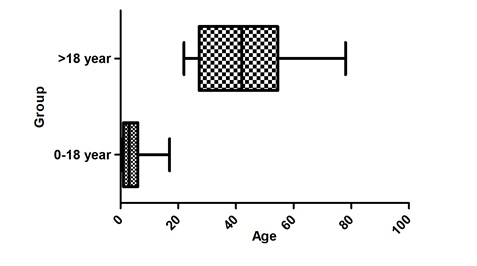 Figure 1: Statistical representation of infection in different patient groups.
Figure 1: Statistical representation of infection in different patient groups.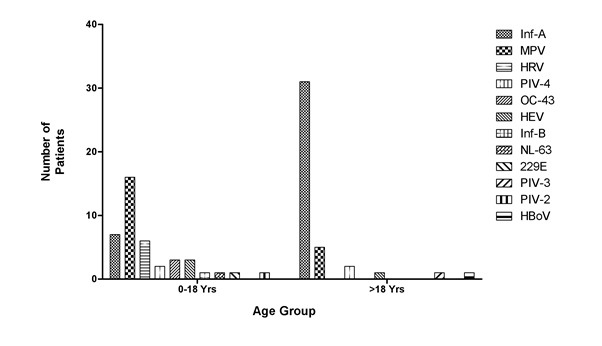 Figure 2: Distribution of respiratory virus in different age groups.
Figure 2: Distribution of respiratory virus in different age groups.Although, the climatic conditions during the study period are same for both the study groups but it was found that the patients below age of 18 year shows maximum severity in terms of symptoms (Figure 3).The rate of respiratory infections were also more frequent in below 18 year age group (Figures 4.1 and 4.2).
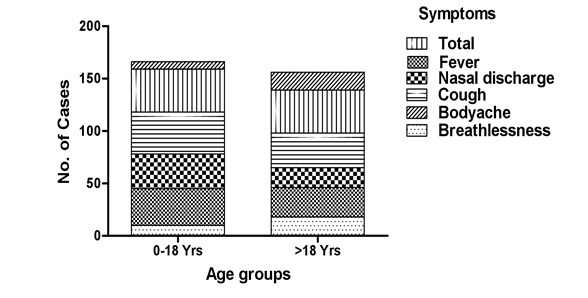
Figure 3: Symptomatic variations among different age groups.
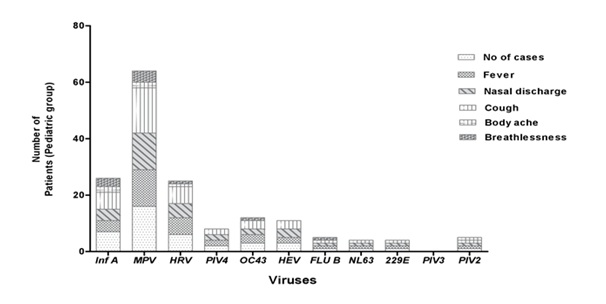
Figure 4.1: Comparison of different virus infection rates in patients of 0-18 years age group.
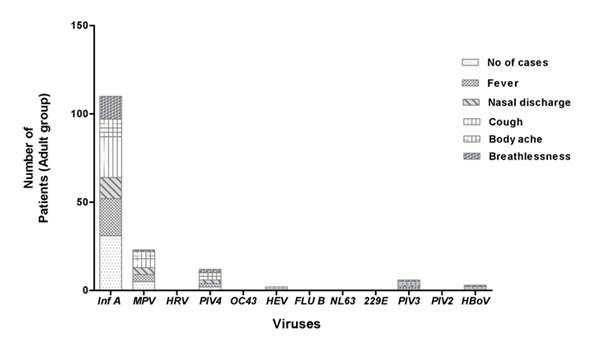
Figure 4.2: Comparison of different virus infection rates in patients above 18 years.
Also, the study reveals that the extensive pathogen infecting patients above 18 years, age group is Influenza A (71.6%) while it is Metapneumovirus (39.02%) for patients below 18 age group (Figure 2).
DISCUSSION
The viruses continue to pose novel challenges by donning new disguises that manage to outwit our immune system. The data on the clinical presentations of respiratory viruses needs to be extensively studied. This surveillance data confirms the virulence and strain circulatory patterns in different age groups in Delhi.
Although both the age groups were reported to have respiratory viral infection, however, our findings show that the group ranging 0-18 years was found to be infected more frequently as compared to other older groups (Figures 4.1and 4.2). The result depicts that only the patients below 18 years, was more prone to infection by different respiratory viral flora rather than the other patient group (>18years). The young age which might acts as a substrate factor reflecting the reciprocity of factors causing disease following viral infection. The severity of infection can be contributed to the age of child which has an effect in terms of the airway size, transmission dynamics, and immune experience of the child. As younger children have smaller energy reserves, they tends to get exhausted by the effort during breathing, which can be concluded for mortality in acute bronchiolitis. There are also critical differences in the infant immune system compared to that of adults that directly affect infection [40].
ILI can be attributed to a wide range of respiratory viruses, including influenza viruses, adenoviruses, RSV, HEV, HRV, hMPV, HBoV, CoV, and PIV. Patients infected by these diverse viral pathogens develop similar symptoms which transcribe unreliable clinical diagnosis and limit etio-epidemiological studies [41].
The proportion of infectivity for both influenza virus and metapneumovirus were quite distinguishable and reveals that, the both virus caused maximum illness, in all age group as it includes higher rates of reported cough, breathlessness and bodyache to the patients (Figure 3) .The higher percentage of symptomatic cough in viral infection may indicate substantial cellular damage to the respiratory epithelium. The ?rst days of a viral URTI are often associated with a dry, unproductive cough, which may be caused by the in?ammatory response in the upper airways spreading to the larynx and cause loss of sleep and exhaustion. Cough associated with URTIs is believed to be caused by a hyper-reactivity of the cough re?ex that may be due to the effects of in?ammatory mediators on airway sensory nerve endings [42]. Cough occurs spontaneously with an URTI, and some cough may be voluntary rather than re?ex; this voluntary cough may be related to a sensation of airway irritation [43]. Productive cough usually occurs later in the course of URTI and may be related to the in?ammation spreading to the lower airways and triggering mucus production.
The high rates of breathlessness and bodyache is contributed to the formation of free radical and proinflammatory cytokines which ultimately cause oxidative stress during viral infection. In context to free radical formed in airway nitric oxide (NO) is merely important and is almost unreactive radical except for its termination reaction with superoxide radical to yield the strong oxidant peroxynitrite. Excess of NO species assumed to be directly related to hypotension and septic shock and endotoxemia to produce muscle dysfunction and oxidative stress. Myalgia is a common symptom of URTIs, contributing 50% of common cold in patients [44,45]. Fever associated with URTIs is usually accompanied by other systemic symptoms such as myalgia, indicates that both these symptoms are caused by the production of prostaglandin E2 in response to circulating cytokines [46,47].
These findings show the extent to which a respiratory virus put burden among the different age. Early diagnosis improves the efficiency of prophylaxis or treatment by antivirals and has a strong impact on the cost–effectiveness of curative treatment. A microbiological diagnosis may have a significant impact on decreasing excessive and inappropriate use of antibiotics [48]. Excessive antibiotic use is being increasingly recognized as the main selective pressure driving resistance. In a study investigating outpatient antibiotic use in 26 countries in Europe, there was a shift from the old narrow-spectrum antibiotics to the new broad-spectrum antibiotics and there were higher rates of antibiotic resistance in higher consuming countries [49]. One of the consequences of over-prescribing antibiotics, especially to individuals with viral infections only, is that no clinical improvement is observed and the potential risk to cause antibiotic resistance increases. As new antiviral options become available, better treatment choices will benefit the patient and community. Molecular techniques provide timely information regarding the identification of pathogens detected and will guide the clinician’s choice of therapy [50].
The observations documented in the present study provide an insight to the epidemiology and clinical manifestation of respiratory viruses in the common population in northern India and may help clinicians in making an early diagnosis, help to understand the roles that pathogens play in particular infection so as to institute appropriate treatment and management. Also, the counseling should be instituted through clinicians or health care workers to the patients and their family.
Although both the age groups were reported to have respiratory viral infection, however, our findings show that the group ranging 0-18 years was found to be infected more frequently as compared to other older groups (Figures 4.1and 4.2). The result depicts that only the patients below 18 years, was more prone to infection by different respiratory viral flora rather than the other patient group (>18years). The young age which might acts as a substrate factor reflecting the reciprocity of factors causing disease following viral infection. The severity of infection can be contributed to the age of child which has an effect in terms of the airway size, transmission dynamics, and immune experience of the child. As younger children have smaller energy reserves, they tends to get exhausted by the effort during breathing, which can be concluded for mortality in acute bronchiolitis. There are also critical differences in the infant immune system compared to that of adults that directly affect infection [40].
ILI can be attributed to a wide range of respiratory viruses, including influenza viruses, adenoviruses, RSV, HEV, HRV, hMPV, HBoV, CoV, and PIV. Patients infected by these diverse viral pathogens develop similar symptoms which transcribe unreliable clinical diagnosis and limit etio-epidemiological studies [41].
The proportion of infectivity for both influenza virus and metapneumovirus were quite distinguishable and reveals that, the both virus caused maximum illness, in all age group as it includes higher rates of reported cough, breathlessness and bodyache to the patients (Figure 3) .The higher percentage of symptomatic cough in viral infection may indicate substantial cellular damage to the respiratory epithelium. The ?rst days of a viral URTI are often associated with a dry, unproductive cough, which may be caused by the in?ammatory response in the upper airways spreading to the larynx and cause loss of sleep and exhaustion. Cough associated with URTIs is believed to be caused by a hyper-reactivity of the cough re?ex that may be due to the effects of in?ammatory mediators on airway sensory nerve endings [42]. Cough occurs spontaneously with an URTI, and some cough may be voluntary rather than re?ex; this voluntary cough may be related to a sensation of airway irritation [43]. Productive cough usually occurs later in the course of URTI and may be related to the in?ammation spreading to the lower airways and triggering mucus production.
The high rates of breathlessness and bodyache is contributed to the formation of free radical and proinflammatory cytokines which ultimately cause oxidative stress during viral infection. In context to free radical formed in airway nitric oxide (NO) is merely important and is almost unreactive radical except for its termination reaction with superoxide radical to yield the strong oxidant peroxynitrite. Excess of NO species assumed to be directly related to hypotension and septic shock and endotoxemia to produce muscle dysfunction and oxidative stress. Myalgia is a common symptom of URTIs, contributing 50% of common cold in patients [44,45]. Fever associated with URTIs is usually accompanied by other systemic symptoms such as myalgia, indicates that both these symptoms are caused by the production of prostaglandin E2 in response to circulating cytokines [46,47].
These findings show the extent to which a respiratory virus put burden among the different age. Early diagnosis improves the efficiency of prophylaxis or treatment by antivirals and has a strong impact on the cost–effectiveness of curative treatment. A microbiological diagnosis may have a significant impact on decreasing excessive and inappropriate use of antibiotics [48]. Excessive antibiotic use is being increasingly recognized as the main selective pressure driving resistance. In a study investigating outpatient antibiotic use in 26 countries in Europe, there was a shift from the old narrow-spectrum antibiotics to the new broad-spectrum antibiotics and there were higher rates of antibiotic resistance in higher consuming countries [49]. One of the consequences of over-prescribing antibiotics, especially to individuals with viral infections only, is that no clinical improvement is observed and the potential risk to cause antibiotic resistance increases. As new antiviral options become available, better treatment choices will benefit the patient and community. Molecular techniques provide timely information regarding the identification of pathogens detected and will guide the clinician’s choice of therapy [50].
The observations documented in the present study provide an insight to the epidemiology and clinical manifestation of respiratory viruses in the common population in northern India and may help clinicians in making an early diagnosis, help to understand the roles that pathogens play in particular infection so as to institute appropriate treatment and management. Also, the counseling should be instituted through clinicians or health care workers to the patients and their family.
ACKNOWLEDGEMENT
The authors acknowledges Defense Research and Development Organization, Govt. of India for the financial support.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Khanna M, Saxena L, Rajput R, Kumar B, Prasad R (2015) Gene silencing: a therapeutic approach to combat influenza virus infections. Future Microbiol 10: 131-140.
- Kumar B, Rajput R, Pati DR, Khanna M (2015) Potent Intracellular Knock-Down of Influenza A Virus M2 Gene Transcript by DNAzymes Considerably Reduces Viral Replication in Host Cells. Mol Biotechnol 57: 836-845.
- Khanna M, Sharma S, Kumar B, Rajput R (2014) Protective Immunity Based on the Conserved Hemagglutinin Stalk Domain and Its Prospects for Universal Influenza Vaccine Development. BioMed Res Inter: 546274.
- Bitko V, Barik S (2007) Respiratory viral diseases: access to RNA interference therapy. Drug discovery today Therapeutic strategies 4: 273-276.
- Kumar B, Asha K, Khanna M, Ronsard L, Meseko CA, et al. (2018) The emerging influenza virus threat: status and new prospects for its therapy and control. Arch Virol 163: 831-844.
- Khanna M, Kumar P, Choudhary K, Kumar B, Vijayan VK (2008) Emerging influenza virus: a global threat. J Biosci 33: 475-482.
- Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C (2002) Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2: 25-32.
- Singh SK (2014) Human respiratory viral infections. CRC press, New York: 164.
- Asner SA, Petrich A, Hamid JS, Mertz D, Richardson SE, et al. (2014) Clinical severity of rhinovirus/enterovirus compared to other respiratory viruses in children. Influenza Other Respir Vir 8: 436-442.
- Sun B, He H, Wang Z, Qu J, Li X, et al. (2014) Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care 18: 456.
- Mahony JB (2008) Detection of Respiratory Viruses by Molecular Methods. Clin Microbiol Rev 4: 716-747.
- Atmar RL (2007) Influenza viruses. Manual of clinical microbiology. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (9th Ed.). ASM Press, Washington, DC: 1340-1351.
- Strelkauskas A, Strelkauskas J, Moszyk-Strelkauskas D (2009) Microbiology: A Clinical Approach. Garland Science, New York.
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, et al. (2011) Harrison's Principles of Internal Medicine (18th Ed.). McGraw Hill Professional: 1491.
- Zander DS, Farver CF (2008) Pulmonary Pathology: A Volume in Foundations in Diagnostic Pathology Series. Elsevier Health Sciences, Churchill, Livingstone.
- Parthasarathy A (2013) Textbook of Pediatric Infectious Diseases. JP Medical Publishers Ltd., New Delhi : 138-139.
- Van den Hoogen BG, Van Doornum GJJ, Fockens JC, Cornelissen JJ, Beyer WEP, et al. (2003) Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis 188: 1571-1577.
- Mahony JB, Petrich A, Smieja M (2011) Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci 48: 217–249.
- Mandell L, Woodhead M, Ewig S, Torres A (2006) Respiratory Infections. CRC Press, New York: 186.
- Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM (2006) Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 78: 1232-1240.
- Chung JY, Han TH, Kim SW, Hwang ES (2007) Respiratory picornavirus infections in Korean children with lower respiratory tract infections. Scand J Infect Dis 39: 250-254.
- Druce J, Tran T, Kelly H, Kaye M, Chibo D, et al. (2005) Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002-2003. J Med Virol 75: 122-129.
- Freymuth F, Vabret A, Cuvillon-Nimal D, Simon S, Dina J, et al. (2006) Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol 78: 1498-1504.
- Loens K, Goossens H, de Laat C, Foolen H, Oudshoorn P, et al. (2006) Detection of rhinoviruses by tissue culture and two independent amplification techniques, nucleic acid sequence-based amplification and reverse transcription-PCR, in children with acute respiratory infections during a winter season. J Clin Microbiol 44: 166-171.
- Miller EK, Xiaoyan Lu, Erdman DD, Poehling KA, Zhu Y, et al. (2007) Rhinovirus- associated hospitalizations in young children. J Infect Dis 195: 773-781.
- Romero JR (2007) Enteroviruses and parechoviruses. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (9th Ed.) Manual of clinical microbiology. ASM Press, Washington, DC: 1392-1404.
- Hsiung GD (1973) Enteroviruses. In: Hsiung GD. Diagnostic virology. Yale University Press, New Haven, CT: 54-67.
- Melnick JL, Wenner HA, Phillips CA (1979) Enteroviruses. In: Lennette EH, Schmidt NJ (5th Ed.). Diagnostic procedures for viral, rickettsial and chlamydia infections. American Public Health Association, Washington, DC: 471-534.
- Benschop KSM, Schinkel J, Luken ME, van den Broek PJM, Beersma MFC, et al. (2006) Fourth human parechovirus serotype. Emerg Infect Dis 12: 1572-1575.
- Cherry JD (2004) Enteroviruses and parechoviruses. In: Feigin RD, Cherry JD, Demmler G, Kaplan SL (5th Ed.) Textbook of pediatric infectious diseases. Saunders press, Philadelphia, PA: 1984-2041.
- Vallet S, Gagneur A, Talbot PJ, Legrand MC, Sizun J, et al. (2004) Detection of human coronavirus 229E in nasal specimens in large scale studies using an RT-PCR hybridization assay. Mol Cell Probes 18: 75-80.
- Falsey AR, Walsh EE, Hayden FG (2002) Rhinovirus and coronavirus infection-associated hospitalization among older adults. J Infect Dis 185: 1338-1341.
- Pene F, Merlat A, Vabret A, Rozenberg F, Buzyn A, et al. (2003) Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis 37: 929-932.
- Simon A, Völz S, Höfling K, Kehl A, Tillman R, et al. (2007) Acute life threatening event (ALTE) in an infant with human coronavirus HcoV- 229E infection. Pediatr Pulmonol 42: 393-396.
- Smuts H, Hardie D (2006) Human Bocavirus in Hospitalized Children, South Africa. Emerg Infect Dis 12: 1457-1458.
- Pati DR, Khanna M, Kumar B, Kumar P, Rajput Ret al. (2003) Clinical presentation of patients with seasonal influenza and pandemic influenza a (H1N1-2009) requiring hospitalization. Indian J Chest Dis Allied Sci 55: 15-19.
- Kumar P, Kumar B, Gupta A, Sharma B, Vijayan VK, et al. (2010) Diagnosis of Novel Pandemic Influenza Virus 2009 H1N1 in Hospitalized Patients. Indian J Virol 21: 45-49.
- Kumar B, Kumar P, Rajput R, MK Daga, Singh V, et al. (2012) Comparative reproducibility of SYBR Green I and TaqMan real-time PCR chemistries for the analysis of matrix and hemagglutinin genes of Influenza A viruses. Int J Collab Res Intern Med Public Health 4: 1-7.
- Kumar B, Pati DR, Khanna M, Kumar P, Daga MK, et al. (2012) Age-Sex Distribution and Seasonality Pattern among Influenza Virus Infected Patients in Delhi, 2009-2010. Indian J Community Med 37: 57-58.
- Tregoning JS, Schwarze J (2010) Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology and Immunology. Clin Microbiol Rev 23: 74-98.
- Laguna-Torres A, Gómez J, Ocaña V, Aguilar P, Saldarriaga T, et al. (2009) Influenza-like illness sentinel surveillance in Peru. PLoS One 4: e6118.
- Eccles R, Weber O (2009) Common cold. Biomedicine, Springer Science and Business Media.
- Eccles R (2009) Central mechanisms IV: conscious control of cough and the placebo effect. Handb Exp Pharmacol 187: 241-62.
- Wolfgang S, Martin M, Peter R (2010) Inhibition of nitric oxide synthase during sepsis: revival because of isoform selectivity?. Shock 34: 321-322.
- Eccles R, Loose I, Jawad M, Nyman L (2003) Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med 4: 118-124.
- Baracos V, Rodemann HP, Dinarello CA, Goldberg AL (1983) Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 308: 553-558.
- Ferreira SH (1986) Prostaglandins, pain, and in?ammation. Agents Actions Suppl 19: 91-98.
- Ogrin BJ, Klepser ME (2013) Rapid Diagnostic Tests and Antibiotic Prescribing. Pharmaceut Anal Acta 4: 206.
- Goossens H, Ferech M, Vander SR, Elseviers M (2005) Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365: 579-587.
- Templeton KE (2007) Why diagnose respiratory viral infection? J Clin Virol 40: S2-S4.
Citation: Pati DR, Sharma S, Kumar B, Singh V, Khanna M(2019) Evaluation of respiratory virus pathogen from patients presenting with influenza like illness at various hospitals in Delhi, India. J Pulm Med Respir Res 5: 023.
Copyright: © 2019 Dibya Ranjan Pati, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

