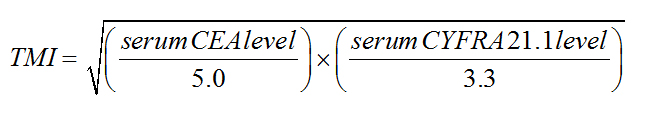Introduction
Approximately 80% of lung cancers are Non-Small Cell Lung Cancer (NSCLC). Improved screening using thoracic computed tomography increased early diagnosis of Stage I NSCLC, as well as, the 5-year survival after curative surgery [1-4]. Despite optimal surgical treatment, cancer recurs in >20.0% of patients. The median post-recurrence survival time ranges from 8.1 to 17.7 months[5]. Tumor size, tumor location and morphology, and lymph node involvement, contribute to disease recurrence. The histological heterogeneity of NSCLC with different clinical presentations makes prognosis difficult[6]. Identification of preoperative serum markers enables more accurate prognosis[7]. Carcinoembryonic Antigen (CEA) and Cytokeratin Fragment 19 (CYFRA 21-1) are two predictive markers that have been extensively studied in lung cancer[8].
Oncofetal glycoprotein CEA is overexpressed in approximately 35-60% of patients with NSCLC [9]. A number of studies demonstrates that elevated preoperative serum CEA concentrations predict a poor prognosis in early-stage NSCLC[10-13], while others report no prognostic value for Overall Survival (OS)[14-18], but rather associated with tumor histology, smoking [14,15], or postoperative disease recurrence[16-18].
Cytokeratin 19 is expressed in the epithelial lining of the bronchial tree and is overexpressed in lung tumors [19]. The C-terminal fragment of cytokeratin 19 is detected by two specific monoclonal antibodies (Bm 19.21 and Ks 19.1). Elevated serum CYFRA 21-1 was found in lung cancer patients [20,21]. Serum CYFRA 21-1 levels are independent of sex and smoking history[22]. Several reports have demonstrated the independent prognostic value of CYFRA 21-1 for survival and tumor relapse after resection[23-26].
Studies that evaluated the prognostic significance of CEA and CYFRA 21-1in the same patient population with stage I-II NSCLC suggested that only CYFRA 21-1, and not CEA, was an independent prognostic indicator for survival [27] and metastasis[28], while others[29,30], showed that both markers have independent prognostic value.It was also noted that prognosis was dependent on tumor histology: patients with adenocarcinoma, but not squamous cell carcinoma had elevated preoperative CEA associated with shorter survival and early recurrence.In contrast, poor prognosis in squamous cell carcinoma was associated with elevated CYFRA 21-1[31]. Higher serum CEA concentrations in adenocarcinoma patients made it difficult to estimate the CEA cutoff value [32,33].
The combination of tumor markers significantly improves their prognostic ability. Cedrés et al. showed that patients with Stage III–IV NSCLC with elevated three markers, CEA, CYFRA 21-1, and cancer antigen-125,had the poorest prognosis[33]. A similar result was obtained using a combination of CEA, CYFRA 21-1, and neuron-specific enolase [34]. A combination of CYFRA 21-1 and a laminin chain gamma 2, predicted a poor OS better than any other markers associated with NSCLC[35]. The CEA and CYFRA 21-1 combination is also reported to have higher sensitivity and specificity for the clinical diagnosis of NSCLC [36-38]. CEA and CYFRA 21-1 gene expression signature was also predictive of a favorable outcome [39].
Muley et al. have shown that a geometric mean of the preoperative CEA and CYFRA 21-1 levels normalized to their respective diagnostic cutoff values, called tumor marker index (TMI), has a prognostic significance[40].The same authors [41] and subsequently Tomita et al. [42] later confirmed these findings, while Blankenburg et al. [43] found no association between elevated preoperative TMI and a poor postoperative prognosis.
To resolve this controversy, this study investigates the usefulness of the TMI in a large patient population with equal number of men and women. We demonstrate that TMI is an independent prognostic factor for postoperative 5-year OS and disease-free survival (DFS) in patients with Stage I NSCLC.
Materials and Methods
Subjects
All study participants have provided informed written consent. The study was approved by the Ethics Committee Ethics Committee of Tokyo Medical University (Tokyo, Japan). The research was conducted in accordance with the Declaration of Helsinki.
This retrospective study included 454 patients with Stage I NSCLC who underwent complete surgical resection, consisting of a lobectomy and lobe-specific selective lymph node dissection, at Tokyo Medical University Hospital between January 2006 and December 2009. Lymph node dissection was performed in all cases. The cohort comprised 252 men and 202 women, with a median age of 67 (range: 35-86) years. Patients were followed up for ≥5 years or until death. The overall postoperative follow-up period ranged from 46 to 2,955 days (1.5-97.2 months), with a mean of 2,004 days or 65.9 months.
Tumor size and histology
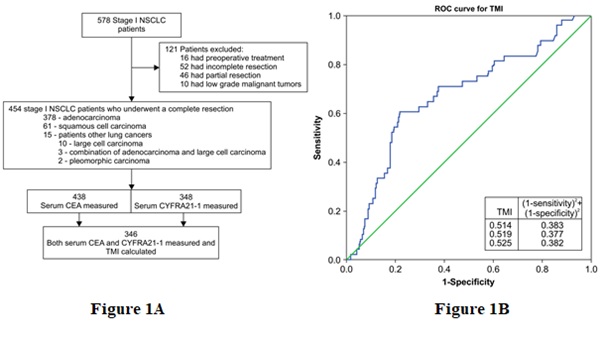
Histological evaluations were performed at our hospital’s Department of Surgical Pathology. Two independent pathologists specializing in lungs and other mediastinal organs performed the histological evaluations. Among the 454 patients, 378 had adenocarcinoma, 61 had squamous cell carcinoma, and 15 had other lung cancers (large cell carcinoma [n=10], adenocarcinoma with large cell carcinoma [n = 3], and pleomorphic carcinoma [n=2]). Patients who underwent preoperative treatment, as well as, those with incomplete or partial resection (e.g., segmentectomy) and patients with low-grade malignant tumors (e.g., carcinoid or mucoepidermoid carcinomas) were excluded from the study (Figure 1A).
Carcinoembryonic antigen and cytokeratin-19 fragment measurements
Preoperative serum CEA and CYFRA 21-1 levels were measured in the in-house hospital clinical laboratory in 448 and 348 patients, respectively. The normal upper limits for serum CEA and CYFRA 21-1 levels were 5.0 and 3.3 ng/mL, respectively. The TMI was calculated as the geometric mean of the CEA and CYFRA 21-1 levels normalized to their respective diagnostic cutoff values (5.0 and 3.3 ng/mL, respectively) in 346 patients in whom both markers had been measured, using the following equation [40].
Statistical analyses
Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal TMI cutoff value for predicting probable OS and DFS rates. The point with the greatest combined specificity and sensitivity was chosen according to the area under the ROC curve analysis (0.675 [95.0% confidence interval]: 0.591-0.760). The optimal TMI cutoff value for predicting postoperative survival was 0.5 according to min (1-sensitivity)2 + (1-specificity)2 expression that takes both sensitivity and specificity into account (Figure 1B).
Postoperative OS and DFS curves were plotted according to the Kaplan-Meier method. OS was measured from the date of surgery to the date of death or last follow-up. A log-rank test was performed to evaluate the significance of differences in survival rates among the groups. A P<0.05 was considered statistically significant. Multivariate analysis using the Cox proportional hazards model was used to establish the association between tumor size and CEA and CYFRA 21-1 levels and the TMI. Additional characteristics (age, sex, smoking history, and tumor histology) were included in the univariate analysis. All statistical analyses were conducted using Statistical Package for the Social Sciences for Windows (software version 24.0; IBM Corp., Armonk, NY, USA).
Results
Patient description and marker levels
The cohort consisted of 454 patients. The median age prior to surgery was 67 (range: 35-86) years, with 77.8% of patients <75 years. All of the patients had Stage I disease. The patient characteristics are summarized in Table 1.
Preoperative serum CEA levels ranged from 0.2 to 137.0 ng/mL (median: 1.5 ng/mL); 23.1% of patients had elevated CEA levels of >5.0 ng/mL. CYFRA 21-1 levels ranged from 0.1 to 212.0 ng/mL (median: 1.0 ng/mL); 6.6% of patients had elevated CYFRA 21-1 levels of >3.3 ng/mL. In 346 patients, both markers had been measured, and the TMI was calculated. TMI values ranged from 0.09 to 3.87 (median: 0.50); 50.3% of patients had an elevated TMI value of >0.5. Among patients who had normal serum CEA and CYFRA 21-1 levels, 84 had an elevated TMI value. The CEA, CYFRA 21-1, and TMI data are summarized in Table 1.
Survival and univariate and multivariate analyses
The mean postoperative follow-up period was 2,004 days (65.9 months), ranging from 46 to 2,995 days (1.5-97.2 months). The 5-year survival rate was 85.0%.
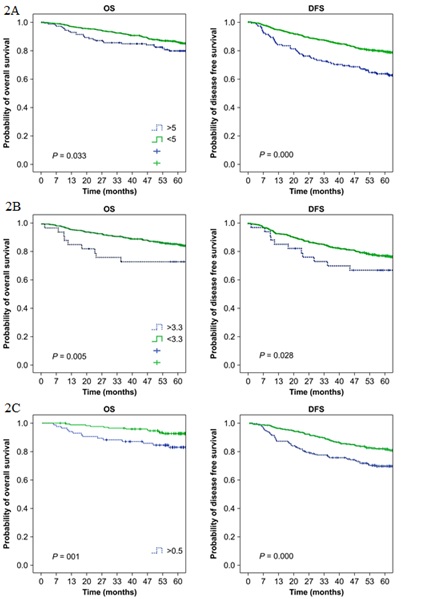
The 5-year OS and DFS rates were significantly lower for patients with elevated serum CEA levels than for patients with normal CEA levels (P<0.01 and P<0.001, respectively) (Figure 2A and Table 2). Similarly, OS and DFS rates were significantly lower for patients with elevated serum CYFRA 21-1 levels than for patients with normal CYFRA 21-1 levels (P<0.01 and P<0.05, respectively) (Figure 2B and Table 2). In addition, significantly lower OS and DFS rates were observed for patients with an elevated TMI value than for patients with a normal TMI value (P<0.001 for both OS and DFS) (Figure 2C and Table 2).
The clinical variables associated with a significantly poorer survival that were identified by univariate analysis included: male sex (mean survival: 1,785 days in men vs. 1,940 days in women; P<0.01), smoking history (1,771 days in smokers vs. 1,972 days in non-smokers; P<0.01), tumor size (1,651 days for tumors >3.0 cm vs. 1,978 days for tumors <3.0 cm; P<0.001), and tumor histology (1,353 days for non-adenocarcinoma vs. 1,794 days for adenocarcinoma; P<0.0001) (Table 3 and Table 4).
Univariate analysis of preoperative tumor markers confirmed their significance for OS (Table 3). The mean survival of patients with serum CEA levels of >5.0 ng/mL was 1,454 days compared to 1,869 days for patients with serum CEA levels of <5.0 ng/mL (P<0.05). The mean survival of patients with serum CYFRA 21-1 levels of >3.3 ng/mL was 1,472 days compared to 1,845 days for patients with serum CYFRA 21-1 levels of <3.3 ng/mL (P<0.01). Patients in whom the calculated TMI value was >0.5 had a lower mean survival than patients with a TMI value of <0.5 (1,702 vs. 1,944 days, respectively; P<0.01).
Multivariate analysis identified the TMI as being an independent prognostic factor not only for OS (hazard ratio: 1.966, 95.0% confidence interval: 1.031–3.752; P<0.05), but also for DFS (hazard ratio: 1.563, 95.0% confidence interval: 1.125-2.172; P<0.01) (Table 4). According to the multivariate analysis, tumor size was an independent prognostic factor for DFS only and not OS.
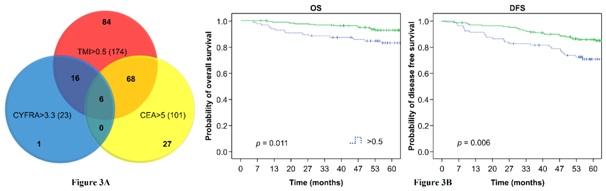
When survival analysis was performed on patients (n=84) who had normal preoperative serum CEA and CYFRA 21-1 levels, but an elevated TMI value, significant differences in the OS and DFS rates were found compared to patients who had all three parameters that were normal (Figure 3A). The 5-year OS rate was 82.8% in patients with a TMI value of >0.5 and 92.6% in patients with a TMI value of <0.5 (P<0.05). The 5-year DFS rate was 70.9% in patients with a TMI value of >0.5 and 86.0% in patients with a TMI value of <0.5 (P<0.01) (Figure 3B).
The DFS and OS are significantly different from the patient who had normal preoperative serum CEA and CYFRA211 levels but an elevated TMI (mean DFS and OS are 16111 days and 1737days), the patients with low three indicators and the patient with elevated CEA or CYFRA211 and TMI (mean DFS and OS are 1383 days and 1461 days).
Discussion
Surgery provides the best option for long-term survival in Stage I–II NSCLC[44]. In addition, adjuvant platinum-based chemotherapy has been shown to be beneficial, especiallyfor patients with Stage II NSCLC [45]. A high incidence of disease recurrence in Stage I patients after complete resection suggests that these patients also might be candidates for adjuvant chemotherapy. Although there are many demographic, clinical, genetic, and pathological parameters that should be taken into consideration during decision making for postoperative chemotherapy, a simple preoperative prognostic test that could overcome the heterogeneous clinical and morphological presentations of NSCLC should be developed. Herein, we present evidence that the TMI, a calculated parameter based on preoperative serum CEA and CYFRA 21-1 levels, is a valid and independent prognostic indicator not only for postoperative OS, but also for DFS.
In accordance with several previous reports [5,6] our results demonstrate that male sex, smoking history, tumor size and histology are independent prognostic factors in NSCLC. We validated the significance of the TMI for the prediction of OS in a much larger cohort than those of previous studies[40-43]. The number of patients included in the study was 454 compared to 153, 261, and 293 patients in previous studies. Furthermore, our patient population consisted of an almost equal number of men and women (252 vs. 202). This is important given the sex differences in survival in patients with NSCLC[46].
All patients included in this study underwent lobe-specific selective lymph node dissection (known as ND2a-1 in Japan), which results in high OS[47]. In our study5-year survival rates were approximately 80.0%. Moreover, the proportion of patients with large cell carcinoma, who usually have a poor prognosis, was relatively low in our study (<20.0%). Despite high survival rates, we were able to demonstrate the effectiveness of the TMI in predicting a poor prognosis. In a previous study, Blankenburg et al. reported that the survival rates in 40.0% of patients with large cell carcinoma were <55.0%. The low OS rates may explain their inability to demonstrate the prognostic significance of the TMI[43].
To further investigate the prognostic value of the TMI, we plotted Kaplan-Meier curves of DFS according to the TMI (Figure 2C and Table 2) and performed univariate and multivariate analyses (Table 4). Our data demonstrated that the TMI is an independent prognostic indicator not only for OS but also for DFS, suggesting that it could be utilized for the prediction of potential disease recurrence, and thus could contribute to the decision making for postoperative chemotherapy.
Our study for the first time demonstrates advantage of TMI compared serum CEA and CYFRA 21-1 levels separately. We show that individual CEA and CYFRA 21-1 measurements often produce non-overlapping results in the same patient population. For example, among 101 patients with elevated serum CEA levels and 23 patients with elevated CYFRA 21-1 levels, the levels of both markers were simultaneously elevated in only 6 patients. Similarly, among 325 patients with normal CYFRA 21-1 levels, 95 patients had elevated serum CEA levels. Furthermore, we found that among 174 patients with an elevated TMI value, 84 had normal CEA and CYFRA 21-1 levels (Figure 3A).
We standardized the prognostic threshold of the TMI as 0.5 using ROC analysis. Previous studies [40-43], used a critical level approach that resulted in various TMI cutoff values for the prediction of survival, complicating the comparison of results between different reports. For instance, Blankenburg et al. defined several prognostic cutoff values for the TMI based on survival curves[43]. They also used a different diagnostic threshold for CEA assay, which may explain the discrepancy between their results and our own.
In accordance with our findings, the TMI has been shown to correlate with imaging characteristics of high-resolution computed tomography, predicting a poor prognosis in early-stage lung adenocarcinoma[47]. Elevated TMI also correlated with a poor survival in aggressive adenosquamous lung carcinoma with a relatively high frequency of EGFR mutations[48].
The TMI has also been calculated for different tumor markers, e.g. for preoperative CEA and Krebs von den Lungen-6 levels in NSCLC[49]. In general, the utilization of a combination of several markers appears to be a more advantageous approach for the diagnosis and assessment of chemotherapy response in lung cancer and other cancer types[50]. For example, a comparison of the diagnostic sensitivity of various combinations of serum tumor markers, including CEA and CYFRA 21-1, in patients with esophageal squamous cell carcinoma suggested that combinations of markers that included Chitinase-3-like-1 protein and squamous cell carcinoma antigen had better diagnostic capabilities than CEA and CYFRA 21-1 for this type of cancer[51]. The usefulness of a prognostic marker index that is calculated as a combination of preoperative serum CEA and plasma tissue inhibitor of metalloproteinases-1 levels for the assessment of the risk of disease recurrence has been demonstrated in patients with early-stage colorectal cancer[52].
Several limitations of our study should be considered. First, false-positive results associated with elevated CEA levels due to smoking could not be excluded[53]. Moreover, this retrospective study did not include the findings of a preoperative clinical examination of the patients, which could influence serum CEA levels. Second, variations in the rates of CEA and CYFRA 21-1 synthesis and elimination in highly heterogeneous lung tumors were not taken into consideration, and third, tumor depth and nodal status were not examined.
Conclusion
The TMI based on preoperative serum CEA and CYFRA 21-1 levels is an independent predictor of a poor prognosis in early-stage NSCLC. Strict follow-up and consideration for postoperative adjuvant chemotherapy is suggested for patients with elevated TMI values. Clinical trials testing the association between TMI values and disease recurrence, as well as, the effectiveness of adjuvant chemotherapy in patients with elevated TMI values should further confirm our findings.
Disclosure statement
The authors have no conflicts of interest.
Source(s) of support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Editorial support, in the form of medical writing, assembling tables, and creating high-resolution images based on the authors’ detailed instructions, collating author comments, copyediting, fact checking, and referencing, was provided by Cactus Communications.