
Journal of Pulmonary Medicine & Respiratory Research Category: Medical
Type: Research Article
The Effects of Internal Airway Percussion (IAP) New Device on the Concentration of Exhaled Protein in Healthy Humans and Canines
*Corresponding Author(s):
Paul W DavenportDepartment Of Physiological Sciences, University Of Florida, Gainesville, Florida, United States
Tel:+352 2944025,
Fax:+352 3925145
Email:pdavenpo@ufl.edu
Received Date: Apr 27, 2016
Accepted Date: Jul 11, 2016
Published Date: Jul 25, 2016
Abstract
BackgroundNon-invasive exhaled breath analysis is a safe and highly efficient strategy for the diagnosis of respiratory diseases but is limited by low concentration of targeted substances in exhaled breath. We utilized a new Internal Airway Percussion (IAP) device that vibrates the air inside of the lungs and airways via the mouth, thereby increase the motion of lung substances, and cause material lining the airways to be aerosolized. We hypothesized IAP would increase the content of protein in exhaled breath samples.
MethodsIAP is a new device that generates a constant frequency square wave that would superimposes on tidal volume to create an internal percussion in the lungs and airways. Seventeen conscious healthy human participants and seven anesthetized dogs were performed with (IAP trial) and without IAP (control trial) via the mouth in this experiment. Exhaled breath samples were collected by filters for 20 minutes with (IAP trial) or without (control trial) 15 Hz square-wave IAP in human participants. Collecting time was reduced to 10 minutes in each trial in dogs. Proteins trapped by the filters were extracted and analyzed by a protein quantization kit. Respiratory perception was estimated by five human participants (n=5) in the 15 Hz IAP groups after each trial.
ResultsThe results showed that 15 Hz IAP increased protein concentration in exhaled breath samples in conscious humans (48%) and anesthetized dogs (32%). In addition, 15 Hz.
IAP significantly increased mean breathing frequency (p<0.05) compared to the control trial. There were no significant changes in respiratory perception between IAP and control trials.
ConclusionsThese results suggest that 15 Hz, square-wave IAP might have potential to increase release of proteins from the respiratory tract into the exhaled airstream without causing respiratory discomfort.
MethodsIAP is a new device that generates a constant frequency square wave that would superimposes on tidal volume to create an internal percussion in the lungs and airways. Seventeen conscious healthy human participants and seven anesthetized dogs were performed with (IAP trial) and without IAP (control trial) via the mouth in this experiment. Exhaled breath samples were collected by filters for 20 minutes with (IAP trial) or without (control trial) 15 Hz square-wave IAP in human participants. Collecting time was reduced to 10 minutes in each trial in dogs. Proteins trapped by the filters were extracted and analyzed by a protein quantization kit. Respiratory perception was estimated by five human participants (n=5) in the 15 Hz IAP groups after each trial.
ResultsThe results showed that 15 Hz IAP increased protein concentration in exhaled breath samples in conscious humans (48%) and anesthetized dogs (32%). In addition, 15 Hz.
IAP significantly increased mean breathing frequency (p<0.05) compared to the control trial. There were no significant changes in respiratory perception between IAP and control trials.
ConclusionsThese results suggest that 15 Hz, square-wave IAP might have potential to increase release of proteins from the respiratory tract into the exhaled airstream without causing respiratory discomfort.
Keywords
Exhaled air; Internal airway percussion; Respiratory perception
INTRODUCTION
BAL (Bronchoaveolar Lavage) performed during bronchoscopy is commonly used to collect Airway Surface Liquid (ASL) for the identification of interstitial airway and lung pathophysiology [1,2]. It has been demonstrated that there were specific alterations in protein composition of ASL upon different respiratory disorder [3,4]; therefore, analysis of these changes in protein profile may be of value for diagnosing respiratory diseases or monitoring of pathological processes in respiratory tracts. In clinical diagnosis, BAL provides quantitative and specific information on diseases but it is invasive and may cause lung damage or inflammation [5]. It is not recommended to be repeated at frequent intervals and is unsuitable in many seriously ill patients and children [5]; thus, non-invasive diagnostic tools that can provide high resolution of airway and lung pathophysiology are necessary.
Exhaled Breath Condensate (EBC) is a non-invasive tool that has been used to collect substances originating from the respiratory tracts [6-8]. Exhaled breath samples are complex and are from a wide variety of origins throughout the respiratory tract. First are aerosolized particles of ASL that contribute the non-volatile constituents of exhaled breath samples, including ions and proteins. Second are volatile organic compounds that have been widely studied in health and diseases [9-12]. The quantization of these exhaled breath substances have potential for use as biomarkers for the diagnosis of respiratory diseases. For example, angiogenic makers (ex. vascular endothelial growth factor, basic fibroblast growth factor and angiotensin) have been found in EBC in patients with non-small cell lung cancer [13]. In addition, exhaled inflammatory makers (ex. Tumor necrosis factor-α, interleukin 6) have been identified in animals with acute lung injury [14]. Although these biomarkers in EBC have been demonstrated to have specificity for identifying pulmonary diseases and the procedure is more applicable in seriously ill patients and children compared to BAL, it is still not widely used in patients due to poor sensitivity because of low concentrations of protein in the exhaled breath samples during normal tidal breathing. Therefore, it is important to develop methods to increase the content of protein in the exhaled breath for quantitative clinical applications.
Airway percussion has been reported to improve the clearance of excess mucus in Chronic Obstructive Pulmonary Disease (COPD) and cystic fibrosis patients. Intrapulmonary percussive devices, such as Acapella and Flutter rely on the energy from passive exhalation to generate airway oscillations (Acapella: 8-30 Hz; Flutter: 15-29 Hz) that have been used to facilitate secretion removal [13-18]. In addition, high frequency chest wall oscillation (2-25 Hz) consists of an air-pulse generator that produces a high frequency oscillation through a vest transmitted to the airways to loosen bronchial mucus so that the excess mucus can be expelled by patients [19,20]. Intrapulmonary Percussive Ventilation (IPV) generates short and rapid inspiratory flow into the airway opening that causes airway pressure to oscillate between 5 and 35 cm H2O and the airway walls vibrate in synchrony with these oscillations. Oscillation enhances intra-bronchial secretion resulting in excess mucus expelled [19,21]. The vibralung acuousstical precussor (Vibralung) enhance mucus clearance by means of delivery oscillatory sinusoidal sound waves (5-1200 Hz) to the airways via mouthpiece [22]. These oscillation methods have not, however, been applied in combination with EBC to increase the concentration of proteins exhaled from the respiratory tract.
IAP (Internal Airway Percussion) device is a new device that can generate high frequency oscillation that intralumenally mechanically vibrates the air in the respiratory tract via mouth during normal tidal breathing. It has been demonstrated that IAP with 15 Hz square wave would significantly increase the total mass of particles exhaled from the human respiratory system [23]. In the current study, we hypothesized that IAP-generated intraluminal airway vibration would cause particles and droplets in the ASL on the surface of the airways and alveoli to be detached, aerosolized and appear in the exhaled breath samples; hence exhalation through a filter will trap large molecules such as proteins. We also expected that IAP superimposing on tidal breathing would not change participants’ normal breathing pattern, end-tidal CO2, and heart rate and neither cause respiratory discomfort. The goal of this study was to utilize the mechanical effect of IAP to facilitate the movement of airway proteins out from the respiratory tract to the expired airflow stream and thus washed out in exhaled breath for protein analysis. In this initial study, we applied a square-wave IAP at 5 Hz and 15 Hz to conscious human participants’ tidal breathing through a mouthpiece and collected their exhaled protein to test our hypothesis. Furthermore, we tested the effect of IAP on the enhancement of airway protein appearing in exhaled breaths in endotracheal intubated anesthetized dogs to exclude contamination of the filter sample from the oral cavity and further confirm the increased protein was mainly from thesublaryngeal airways.
Exhaled Breath Condensate (EBC) is a non-invasive tool that has been used to collect substances originating from the respiratory tracts [6-8]. Exhaled breath samples are complex and are from a wide variety of origins throughout the respiratory tract. First are aerosolized particles of ASL that contribute the non-volatile constituents of exhaled breath samples, including ions and proteins. Second are volatile organic compounds that have been widely studied in health and diseases [9-12]. The quantization of these exhaled breath substances have potential for use as biomarkers for the diagnosis of respiratory diseases. For example, angiogenic makers (ex. vascular endothelial growth factor, basic fibroblast growth factor and angiotensin) have been found in EBC in patients with non-small cell lung cancer [13]. In addition, exhaled inflammatory makers (ex. Tumor necrosis factor-α, interleukin 6) have been identified in animals with acute lung injury [14]. Although these biomarkers in EBC have been demonstrated to have specificity for identifying pulmonary diseases and the procedure is more applicable in seriously ill patients and children compared to BAL, it is still not widely used in patients due to poor sensitivity because of low concentrations of protein in the exhaled breath samples during normal tidal breathing. Therefore, it is important to develop methods to increase the content of protein in the exhaled breath for quantitative clinical applications.
Airway percussion has been reported to improve the clearance of excess mucus in Chronic Obstructive Pulmonary Disease (COPD) and cystic fibrosis patients. Intrapulmonary percussive devices, such as Acapella and Flutter rely on the energy from passive exhalation to generate airway oscillations (Acapella: 8-30 Hz; Flutter: 15-29 Hz) that have been used to facilitate secretion removal [13-18]. In addition, high frequency chest wall oscillation (2-25 Hz) consists of an air-pulse generator that produces a high frequency oscillation through a vest transmitted to the airways to loosen bronchial mucus so that the excess mucus can be expelled by patients [19,20]. Intrapulmonary Percussive Ventilation (IPV) generates short and rapid inspiratory flow into the airway opening that causes airway pressure to oscillate between 5 and 35 cm H2O and the airway walls vibrate in synchrony with these oscillations. Oscillation enhances intra-bronchial secretion resulting in excess mucus expelled [19,21]. The vibralung acuousstical precussor (Vibralung) enhance mucus clearance by means of delivery oscillatory sinusoidal sound waves (5-1200 Hz) to the airways via mouthpiece [22]. These oscillation methods have not, however, been applied in combination with EBC to increase the concentration of proteins exhaled from the respiratory tract.
IAP (Internal Airway Percussion) device is a new device that can generate high frequency oscillation that intralumenally mechanically vibrates the air in the respiratory tract via mouth during normal tidal breathing. It has been demonstrated that IAP with 15 Hz square wave would significantly increase the total mass of particles exhaled from the human respiratory system [23]. In the current study, we hypothesized that IAP-generated intraluminal airway vibration would cause particles and droplets in the ASL on the surface of the airways and alveoli to be detached, aerosolized and appear in the exhaled breath samples; hence exhalation through a filter will trap large molecules such as proteins. We also expected that IAP superimposing on tidal breathing would not change participants’ normal breathing pattern, end-tidal CO2, and heart rate and neither cause respiratory discomfort. The goal of this study was to utilize the mechanical effect of IAP to facilitate the movement of airway proteins out from the respiratory tract to the expired airflow stream and thus washed out in exhaled breath for protein analysis. In this initial study, we applied a square-wave IAP at 5 Hz and 15 Hz to conscious human participants’ tidal breathing through a mouthpiece and collected their exhaled protein to test our hypothesis. Furthermore, we tested the effect of IAP on the enhancement of airway protein appearing in exhaled breaths in endotracheal intubated anesthetized dogs to exclude contamination of the filter sample from the oral cavity and further confirm the increased protein was mainly from thesublaryngeal airways.
MATERIALS AND METHODS
Conscious human study
Participants: The human study was approved by the Institutional Review Board of the University of Florida. Twenty-two nonsmoking healthy adults with no history of pulmonary or neurological disease participated in the study after providing informed written consent (Table 1).
| No | Age | Height (cm) | Weight (kg) | Gender |
| 1 | 28 | 165 | 68 | F |
| 2 | 25 | 167 | 90 | F |
| 3 | 26 | 162 | 58 | F |
| 4 | 25 | 157 | 47 | F |
| 5 | 29 | 157 | 50 | F |
| 6 | 26 | 162 | 53 | F |
| 7 | 26 | 155 | 46 | F |
| 8 | 43 | 167 | 63 | F |
| 9 | 36 | 165 | 74 | F |
| 10 | 24 | 175 | 65 | F |
| 11 | 23 | 160 | 58 | F |
| 12 | 30 | 167 | 65 | F |
| 13 | 30 | 172 | 72 | M |
| 14 | 33 | 140 | 63 | M |
| 15 | 30 | 182 | 82 | M |
| 16 | 32 | 167 | 63 | M |
| 17 | 32 | 170 | 73 | M |
IAP device:
The IAP device was constructed from an amplifier (MPG1, Marshall Amplification PLC, Bletchley, Milton Keynes, UK) connected to an acoustic wave controller (HPG1, Velleman® Inc., Fort Worth, TX). The acoustic wave controller allowed adjustment of the frequency of the percussion waves. The IAP device delivered acoustic square-waves to the airway with fixed pressure at 1.29 ± 0.10 cm H2O. The IAP device was attached to a breathing circuit with a heat and moisture exchange filter (Smiths Medical ASD, Keene, NH) using a plastic tube. The condensation foam in the heat and moisture exchanger was used as a filter to capture exhaled protein. A separate sterilized heat and moisture exchanger was connected to the breathing circuit for each breathing trial. A resistance (5 cm H2O/L/sec) approximately equal to normal pulmonary resistance was placed at the end of a breathing circuit to promote transfer of the IAP pressure waveform into the airways. The experimental set up is shown in figure 1.
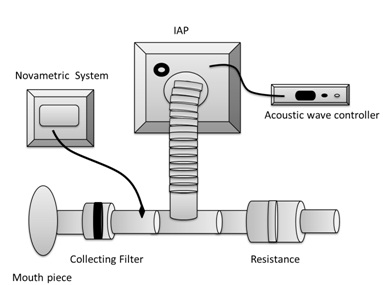 Figure 1: Experimental set up.
Figure 1: Experimental set up.Procedure:
Twenty-two participants were divided into two groups: (A) 5 Hz IAP (n=5) and (B) 15 Hz IAP (n=17). They were seated in a comfortable chair with a nose clip and breathed through a mouthpiece connected to the breathing circuit with a collecting filter. The control trial was breathing with IAP off for 20 minutes. The filter was removed from the circuit and placed in a separate sterilized storage bag. Subjects then were allowed at least a 10 minute break. In the IAP trial, a new filter was inserted into the breathing circuit and the subject again respired through the mouthpiece for 20 minutes with the IAP device activated. The trial order was not randomized because of the potential for the IAP to decrease the normal concentration of proteins in the respiratory tract. Use of IAP prior to non-IAP breathing could result in an IAP trial dependent decreased exhaled protein concentration in the control condition. Thus, the control trial always preceded the IAP trial.
Five participants in the 15 Hz IAP group were asked to estimate the magnitude of their sense of breathing effort, sense of suffocation, sense of air hunger and sense of unpleasantness using modified Borg scales from 0 (=no sensation) to 10 (=maximum) at the beginning of each trial (0 minute), 1 minute after a trial began (1 minute) and immediately after each trail was completed (20 minute).
Five participants in the 15 Hz IAP group were asked to estimate the magnitude of their sense of breathing effort, sense of suffocation, sense of air hunger and sense of unpleasantness using modified Borg scales from 0 (=no sensation) to 10 (=maximum) at the beginning of each trial (0 minute), 1 minute after a trial began (1 minute) and immediately after each trail was completed (20 minute).
Parameters:
End-tidal CO2 (ETCO2), heart rate, respiratory frequency and IAP pressure were recorded during the entire experiment. The signal from monitor was led into a signal processing system (PowerLab, ADI Instruments, Castle Hill, Australia) and a desktop computer for continuous signal recording and analyzed using the LabChart 7 software.
Protein quantitation analysis: Collecting filters were stored separately in a sterilized storage bag at 4? for less than 2 hours for the analysis of protein concentration. The foam was removed from the heat and moisture exchanger filter and placed into a 50 ml conical tube with 10 ml distilled water for 1 hour at room temperature. The sample was vortexed and 100 µl of the solution was used and analyzed by NanoOrange® Protein quantization kit (Invitrogen, Carlsbad, CA) for the protein quantization analysis (Detection range: 10 ng/ml - 10 ug/ml).
Statistical analysis: Mean ETCO2, mean heart rate, mean respiratory frequency and protein concentration were analyzed using one way repeated measures ANOVA. The ratings of breathing effort, suffocation, air hunger and unpleasantness were analyzed with one way repeated measures ANOVA. The significance criterion for all analyses was set at p<0.05.
Anesthetized dog study The study was approved by the University of Florida’s Institutional Animal Care and Use Committee (IACUC). Seven dogs that admitted to the Veterinary Hospital at the University of Florida for a routine dental cleaning were studied. The patient’s medical care was under the supervision of the Veterinary Hospital at the University of Florida. Consent was obtained from the owners prior to the IAP procedure. The dogs were anesthetized and endotracheal intubation performed. Prior to the dental clinical procedure, the IAP breathing circuit and device were connected between the endotracheal tube and an anesthesia machine. The control trial was breathing with IAP off for 10 minutes. Then the IAP breathing circuit was removed between the endotracheal tube and anesthesia machine. The IAP filter was removed from the circuit and placed in a separate sterilized storage bag. The animals were allowed at least a 5 minute rest period. Then the IAP trial was initiated. A new filter was inserted into the breathing circuit and the animal again respired through the filter containing breathing circuit for 10 minutes with the IAP device activated. ETCO2 and heart rate were recorded from the monitor every 30 seconds. The IAP breathing circuit was removed and the clinical procedure performed.
Statistical analysis: Mean ETCO2, mean heart rate and protein concentration were analyzed using one way repeated measures ANOVA. The significance criterion for all analyses was set at p<0.05.
Protein quantitation analysis: Collecting filters were stored separately in a sterilized storage bag at 4? for less than 2 hours for the analysis of protein concentration. The foam was removed from the heat and moisture exchanger filter and placed into a 50 ml conical tube with 10 ml distilled water for 1 hour at room temperature. The sample was vortexed and 100 µl of the solution was used and analyzed by NanoOrange® Protein quantization kit (Invitrogen, Carlsbad, CA) for the protein quantization analysis (Detection range: 10 ng/ml - 10 ug/ml).
Statistical analysis: Mean ETCO2, mean heart rate, mean respiratory frequency and protein concentration were analyzed using one way repeated measures ANOVA. The ratings of breathing effort, suffocation, air hunger and unpleasantness were analyzed with one way repeated measures ANOVA. The significance criterion for all analyses was set at p<0.05.
Anesthetized dog study The study was approved by the University of Florida’s Institutional Animal Care and Use Committee (IACUC). Seven dogs that admitted to the Veterinary Hospital at the University of Florida for a routine dental cleaning were studied. The patient’s medical care was under the supervision of the Veterinary Hospital at the University of Florida. Consent was obtained from the owners prior to the IAP procedure. The dogs were anesthetized and endotracheal intubation performed. Prior to the dental clinical procedure, the IAP breathing circuit and device were connected between the endotracheal tube and an anesthesia machine. The control trial was breathing with IAP off for 10 minutes. Then the IAP breathing circuit was removed between the endotracheal tube and anesthesia machine. The IAP filter was removed from the circuit and placed in a separate sterilized storage bag. The animals were allowed at least a 5 minute rest period. Then the IAP trial was initiated. A new filter was inserted into the breathing circuit and the animal again respired through the filter containing breathing circuit for 10 minutes with the IAP device activated. ETCO2 and heart rate were recorded from the monitor every 30 seconds. The IAP breathing circuit was removed and the clinical procedure performed.
Statistical analysis: Mean ETCO2, mean heart rate and protein concentration were analyzed using one way repeated measures ANOVA. The significance criterion for all analyses was set at p<0.05.
RESULTS
Respiratory physiological parameters
In the conscious human study, the results showed no significant differences in mean ETCO2 and mean heart rate between the control and IAP trials in 5 Hz and 15 Hz IAP groups (Figure 2A and 2B, Table 2). In addition, 15 Hz IAP significantly increased mean breathing frequency (p<0.05) compared to the control trial (Figure 2C, Table 2) that was not found in 5 Hz IAP group.
In the conscious human study, the results showed no significant differences in mean ETCO2 and mean heart rate between the control and IAP trials in 5 Hz and 15 Hz IAP groups (Figure 2A and 2B, Table 2). In addition, 15 Hz IAP significantly increased mean breathing frequency (p<0.05) compared to the control trial (Figure 2C, Table 2) that was not found in 5 Hz IAP group.
 Figure 2: Respiratory parameters in control and IAP trials in conscious humans (A) Mean ETCO2; (B) Mean heart rate (C) Mean respiratory frequency. The * indicates a significant difference, p<0.05. Error bar represents SE
Figure 2: Respiratory parameters in control and IAP trials in conscious humans (A) Mean ETCO2; (B) Mean heart rate (C) Mean respiratory frequency. The * indicates a significant difference, p<0.05. Error bar represents SE| 5 HZ | 15 HZ | |||
| Control | IAP | Control | IAP | |
| ETCO2 (mmHg) | 44.8 ± 3.7 | 46.9 ± 2.4 | 45.1 ± 1.3 | 44.9 ± 1.1 |
| Heart Rate (beat/min) | 78.5 ± 7.3 | 76.3 ± 4.8 | 75.6 ± 1.8 | 74.1 ± 1.6 |
| Respiratory Rate (breath/min) | 16.2 ± 1.3 | 16.7 ± 1.3 | 17.0 ± 1.1 | 18.6 ± 1.2* |
| Exhaled Protein (ug) | 24.6 ± 2.1 | 19.5 ± 1.9* | 10.8 ± 1.7 | 14.5 ± 1.9** |
In anesthetized dogs, there were no significant differences in mean ETCO2 and mean heart rate (Figure 3, Table 3)
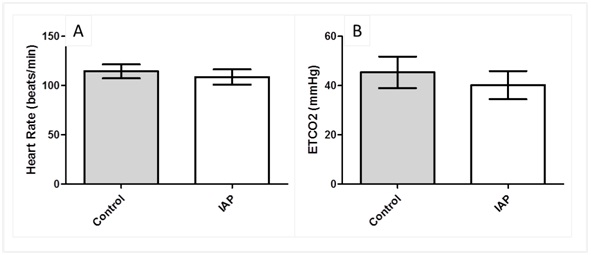
Figure 3: Respiratory parameters in control and IAP trials in dogs (A) Mean ETCO2 (B) Mean heart rate. Error bar represents SE.
| 15 HZ | ||
| Control | IAP | |
| ETCO2 (mmHg) | 45.4 ± 6.4 | 40 ± 5.7 |
| Heart Rate (beat/min) | 114.4 ± 7.1 | 108.7 ± 7.8 |
| Exhaled Protein (ug) | 16.4 ± 4.5 | 20.5 ± 4.7** |
Total quantity of exhaled protein
The total quantity of exhaled protein in each trial was listed in table 2. In conscious humans, square-wave IAP at 5 Hz significantly (p<0.01) decreased the protein concentration by 23% in the exhaled air filters compared to the control trial (Figure 4). In contrast, square-wave IAP at 15 Hz significantly (p<0.01) increased the protein concentration in the exhaled air filters by 48% compared to control trial (Figure 4, Table 2). In anesthetized dogs, square-wave IAP at 15 Hz significantly (p<0.01) increased the protein concentration by 32% in exhaled air filters compared to the control trial (Figure 5, Table 3).
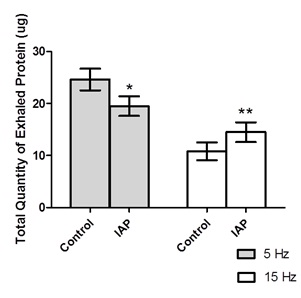 Figure 4: Total quantity of exhaled protein in control and IAP trials in humans. The * indicates a significant difference, p<0.05. The ** indicates a significant difference, p<0.01. Error bar represents SE.
Figure 4: Total quantity of exhaled protein in control and IAP trials in humans. The * indicates a significant difference, p<0.05. The ** indicates a significant difference, p<0.01. Error bar represents SE.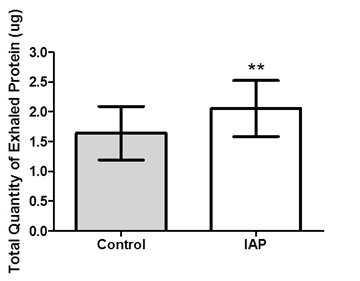 Figure 5: Total quantity of exhaled protein in control and IAP trials in dogs. The ** indicates a significant difference, p<0.01. Error bar represents SE.
Figure 5: Total quantity of exhaled protein in control and IAP trials in dogs. The ** indicates a significant difference, p<0.01. Error bar represents SE.Respiratory perception
Figure 6 shows that the IAP trial did not cause a change in the estimated magnitude of sensation of air hunger, unpleasantness or suffocation compared to control trial in conscious human. However, there was a trend for the magnitude estimation of breathing effort at 20 minutes to decrease with 15 Hz IAP (p=0.07).
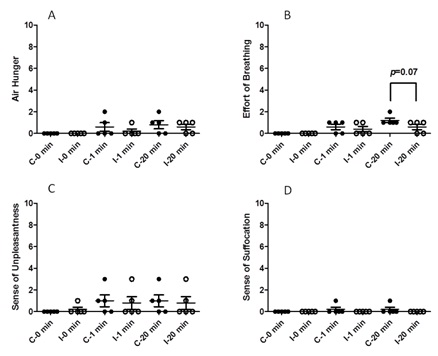
Figure 6: Respiratory perception in humans (A) Air hunger, (B) Effort of breathing, (C) Sense of unpleasantness and (D) Sense of suffocation.
C: Control trial; I: IAP trial. Error bar represents SE.
C: Control trial; I: IAP trial. Error bar represents SE.
DISCUSSION
The main findings of the study are that the 15 Hz, square-wave IAP device could increase the concentration of protein in exhaled breaths in both conscious human participants and anesthetized dogs. In addition, the result showed that 15 Hz IAP significantly increased respiratory frequency compared to control groups. This suggests participants may change their breathing pattern to adapt to the application of IAP, which may contribute to the change in the concentration of exhaled protein. Furthermore, we also tested the subjects’ respiratory sensations during control and IAP trials. We found that IAP did not cause respiratory discomfort in conscious human, such as air hunger, unpleasantness and suffocation and two of five subjects felt they spent less effort on breathing in IAP trial. Thus, the IAP method is well tolerated by the conscious subjects and encourages the subjects to continue using the IAP treatment if needed in repeated trials. This is important for patients and especially for children to encourage their use of the device for diagnosis or monitoring diseases of the respiratory tract.
This effect of IAP on increasing exhaled substances is frequency-dependent. 5 Hz IAP did not increase but decreased the protein concentration in the exhaled air compared to control trials in humans. In contrast, 15 Hz IAP has a greater effect on washing out the substances from respiratory tracts including mouth, tracheobronchial system and alveoli than control trials. Therefore, the frequency of oscillation may play an important role in the percussive effect in the airways, and/or affect the wave propagation into different depths of the airways.
In the present study, the IAP device is an unassisted apparatus that generates sound waves transmitted into airway through a mouthpiece during normal tidal breathing. In contrast to IAP device, Flutter and Acapella are in need of participant passive exhalation to generate chest wall oscillations that the expiratory resistance may be too high for certain serious patients. Therefore, the unassisted IAP device is more applicable in seriously ill patients and children than Flutter and Acapella. In addition, the principles of unassisted IPV and Vibralung operation are similar to IAP devices; however, these similar vibrational techniques are commonly used for the mucus clearance in patients and have not been used for the collection of exhaled substances. The results of the study suggest that IAP has potential to increase exhaled protein that may benefit further research for the diagnosis of respiratory diseases according to exhaled substance profile.
The IAP trials applied to conscious human and anesthetized dogs were different in the isolation of the upper airways and mouth. In the conscious human study, the exhaled protein was collected through a filter connected to a mouthpiece. IAP was applied with the subject breathing through the mouth that may wash out protein from the oral cavity in addition to airways and lungs, affecting the protein quantitative results. However, in the anesthetized dog study, IAP was connected to an endotracheal tube that resulted in the square-wave vibration applied only to sublaryngeal airways and lungs which excludes contamination of the filter sample from the oral cavity for the quantitative analysis. Protein concentration was increased in both study suggesting that the IAP method can increase the amount of protein and other molecules from the lower airways and lung. To exclude the contribution of saliva contamination in the exhaled breath samples, a saliva amylase test needs to be performed in the future studies, although it has been demonstrated that saliva is the source of less than 10% of respiratory droplets [24].
The limitation of the present study was recruiting healthy human as experimental subjects that may not represent the effect of IAP on increasing exhaled substances in patients. In addition, we measured the quantity of total protein in exhaled breath in lack of biomarkers of respiratory disease in healthy subjects that is difficult to state the IAP device would specifically washout certain biomarker for the diagnosis of respiratory diseases. Therefore, further studies need to be done to demonstrate the effect of IAP on increasing biomarkers in exhaled breathe for the diagnosis of specific respiratory diseases.
In conclusion, non-invasive IAP increased the amount of exhaled protein without causing respiratory discomfort. The use of IAP has potential for increasing the sensitivity of exhalate monitoring and diagnosis of respiratory infection, inflammation and other pulmonary diseases.
This effect of IAP on increasing exhaled substances is frequency-dependent. 5 Hz IAP did not increase but decreased the protein concentration in the exhaled air compared to control trials in humans. In contrast, 15 Hz IAP has a greater effect on washing out the substances from respiratory tracts including mouth, tracheobronchial system and alveoli than control trials. Therefore, the frequency of oscillation may play an important role in the percussive effect in the airways, and/or affect the wave propagation into different depths of the airways.
In the present study, the IAP device is an unassisted apparatus that generates sound waves transmitted into airway through a mouthpiece during normal tidal breathing. In contrast to IAP device, Flutter and Acapella are in need of participant passive exhalation to generate chest wall oscillations that the expiratory resistance may be too high for certain serious patients. Therefore, the unassisted IAP device is more applicable in seriously ill patients and children than Flutter and Acapella. In addition, the principles of unassisted IPV and Vibralung operation are similar to IAP devices; however, these similar vibrational techniques are commonly used for the mucus clearance in patients and have not been used for the collection of exhaled substances. The results of the study suggest that IAP has potential to increase exhaled protein that may benefit further research for the diagnosis of respiratory diseases according to exhaled substance profile.
The IAP trials applied to conscious human and anesthetized dogs were different in the isolation of the upper airways and mouth. In the conscious human study, the exhaled protein was collected through a filter connected to a mouthpiece. IAP was applied with the subject breathing through the mouth that may wash out protein from the oral cavity in addition to airways and lungs, affecting the protein quantitative results. However, in the anesthetized dog study, IAP was connected to an endotracheal tube that resulted in the square-wave vibration applied only to sublaryngeal airways and lungs which excludes contamination of the filter sample from the oral cavity for the quantitative analysis. Protein concentration was increased in both study suggesting that the IAP method can increase the amount of protein and other molecules from the lower airways and lung. To exclude the contribution of saliva contamination in the exhaled breath samples, a saliva amylase test needs to be performed in the future studies, although it has been demonstrated that saliva is the source of less than 10% of respiratory droplets [24].
The limitation of the present study was recruiting healthy human as experimental subjects that may not represent the effect of IAP on increasing exhaled substances in patients. In addition, we measured the quantity of total protein in exhaled breath in lack of biomarkers of respiratory disease in healthy subjects that is difficult to state the IAP device would specifically washout certain biomarker for the diagnosis of respiratory diseases. Therefore, further studies need to be done to demonstrate the effect of IAP on increasing biomarkers in exhaled breathe for the diagnosis of specific respiratory diseases.
In conclusion, non-invasive IAP increased the amount of exhaled protein without causing respiratory discomfort. The use of IAP has potential for increasing the sensitivity of exhalate monitoring and diagnosis of respiratory infection, inflammation and other pulmonary diseases.
REFERENCES
- Ali M, Lillehoj EP, Park Y, Kyo Y, Kim KC (2011) Analysis of the proteome of human airway epithelial secretions. Proteome Sci 9: 4.
- Candiano G, Bruschi M, Pedemonte N, Musante L, Ravazzolo R, et al. (2007) Proteomic analysis of the airway surface liquid: modulation by proinflammatory cytokines. Am J Physiol Lung Cell Mol Physiol 292: 185-198.
- Lenz AG, Meyer B, Costabel U, Maier K (1993) Bronchoalveolar Lavage Fluid Proteins in Human Lung-Disease : Analysis by two-Dimensional Electrophoresis. Electrophoresis 14: 242-244.
- Wattiez R, Hermans C, Cruyt C, Bernard A, Falmagne P (2000) Human bronchoalveolar lavage fluid protein two-dimensional database: study of interstitial lung diseases. Electrophoresis 21: 2703-2712.
- de Blic J, Midulla F, Barbato A, Clement A, Dab I, et al. (2000) Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur Respir J 15: 217-231.
- Dwyer TM (2004) Sampling airway surface liquid: non-volatiles in the exhaled breath condensate. Lung 182: 241-250.
- Hunt J (2007) Exhaled breath condensate: an overview. Immunol Allergy Clin North Am 27: 587-596.
- Davis MD, Montpetit A, Hunt J (2012) Exhaled breath condensate: an overview. Immunol Allergy Clin North Am 32: 363-375.
- de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, et al. (2014) A review of the volatiles from the healthy human body. J Breath Res 8: 014001.
- Amann A, Miekisch W, Schubert J, Buszewski B, Ligor T, et al. (2014) Analysis of exhaled breath for disease detection. Annu Rev Anal Chem (Palo Alto Calif) 7: 455-482.
- Haick H, Broza YY, Mochalski P, Ruzsanyi V, Amann A (2014) Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 43: 1423-1449.
- Amann A, Mochalski P, Ruzsanyi V, Broza YY, Haick H (2014) Assessment of the exhalation kinetics of volatile cancer biomarkers based on their physicochemical properties. Journal of Breath Research 8.
- Konstan MW, Stern RC, Doershuk CF (1994) Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J Pediatr 124: 689-693.
- dos Santos AP, Guimaraes RC, de Carvalho EM, Gastaldi AC (2013) Mechanical behaviors of Flutter VRP1, Shaker, and Acapella devices. Respir Care 58: 298-304.
- Volsko TA, DiFiore J, Chatburn RL (2003) Performance comparison of two oscillating positive expiratory pressure devices: Acapella versus Flutter. Respir Care 48: 124-130.
- Pryor JA, Webber BA, Hodson ME, Warner JO (1994) The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. Respir Med 88: 677-681.
- Dasgupta B, Tomkiewicz RP, Boyd WA, Brown NE, King M (1995) Effects of combined treatment with rhDNase and airflow oscillations on spinnability of cystic fibrosis sputum in vitro. Pediatr Pulmonol 20: 78-82.
- Dasgupta B, Brown NE, King M (1998) Effects of sputum oscillations and rhDNase in vitro: a combined approach to treat cystic fibrosis lung disease. Pediatr Pulmonol 26: 250-255.
- Chatburn RL (2007) High-frequency assisted airway clearance. Respiratory Care 52: 1224-1237.
- Chakravorty I, Chahal K, Austin G (2011) A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int J Chron Obstruct Pulmon Dis 6: 693-699.
- Vargas F, Bui HN, Boyer A, Salmi LR, Gbikpi-Benissan G, et al. (2005) Intrapulmonary percussive ventilation in acute exacerbations of COPD patients with mild respiratory acidosis: a randomized controlled trial [ISRCTN17802078]. Crit Care 9: 382-389.
- Mapeck M (2014) Vibralung Acoustical Precursor: A New Paradigm in Airway Clarence Therapy. Respiratory Therapy 9.
- Afshar-Mohajer N, Wu CY, Tsai HW, Silverman E, Davenport P, et al. (2015) Optimizing an Internal Airway Percussion Device for Facilitating Exhalate Diagnostics of the Human Respiratory System. J Aerosol Med Pulm Drug Deliv.
- Effros RM, Peterson B, Casaburi R, Su J, Dunning M, et al. (2005) Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol (1985) 99: 1286-1292.
Citation: Hsiu-Wen Tsai, Condrey J, Adams S, Wuerz J, Specht A, et al. (2016) The Effects of Internal Airway Percussion (IAP) New Device on the Concentration of Exhaled Protein in Healthy Humans and Canines. J Pulm Med Respir Res 2: 006.
Copyright: © 2016 Hsiu-Wen Tsai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

