
Journal of Stem Cells Research Development & Therapy Category: Medical
Type: Short Commentary
Using Transcriptome Analysis for Redefining the in vivo Relevance of in vitro Cultured Circulating Angiogenic Cells (CACiv)
*Corresponding Author(s):
Bert R EveraertLaboratory Of Cell Biology And Histology, Department Of Veterinary Sciences, University Of Antwerp, Antwerp, Belgium
Tel:+3232653300,
Email:berteveraert@me.com, berteveraert@icloud.com
Received Date: Jul 23, 2019
Accepted Date: Aug 19, 2019
Published Date: Aug 26, 2019
Abstract
In a recently published study we demonstrated that in vitro-cultured circulating angiogenic cells (CACiv) are phenotypically similar to regulatory M2c macrophages andshow surprisingly little evidence of endothelial cell differentiation. This almost excludes the hypothesis that CACiv truly undergo transdifferentation towards endothelial cells. Alternatively, we proposed new biological functions by which these CACiv, being M2c macrophages in nature, can interfere with cardiovascular pathophysiology. In this short communication we report the relevance of the CACiv-like gene expression profile in the setting of acute myocardial infarction. This finding validates the CACiv transcriptome as a biologically, in vivo relevant gene expression entity. Rather than indicating active progenitor cell homing we relate this to a local switch from an inflammatory (M1) into a regulatory (M2) macrophage phenotype.
Keywords
inflammatory; macrophage phenotype; Angiogenic Cells
INTRODUCTION
Over the past decades, stem cell research has become a hot topic in several areas of medicine. In cardiovascular medicine the interest in a specific stem cell, i.e. the Endothelial Progenitor Cell (EPC) has been remarkable. Despite the wealth of in vitro and in vivo studies published, valid interpretations and sound, unambiguous conclusions were seriously hampered by the heterogeneity of study methodologies, patient populations, cell culture and enumeration methods and by the absence of a commonly accepted definition of EPCs. Overall, these studies pointed out that EPCs can be regarded as important biomarkers of cardiovascular health; a whole range of (pre) clinically used drugs and lifestyle interventions could offer strategies to intervene with EPC biology in cardiovascular pathology [1]. If indeed EPCs contribute to vascular homeostasis and cardiovascular regenerative processes it seems logical to assume that ischemia-related conditions e.g. myocardial infarction, peripheral artery disease or diabetes induced micro- or macroangiopathy could benefit from the regenerative potential of EPCs.
However, still considerable debate about the origin and function of EPCs remains and much of this uncertainty is caused by a high degree of confusion about the definition of EPCs. Over the years, different culture protocols have emerged, all claiming to produce EPCs from Bone Marrow (BM) or peripheral blood-derived mononuclear cells. Furthermore, a variety of molecular marker combinations have been utilized for their characterization. Obviously, the ambiguity that surrounds the term ‘EPC’ has not facilitated the understanding of EPC biology.
This lack of phenotypical characterization and the limited knowledge of the underlying EPC biology have, however, not hindered the early use of Bone Marrow-Derived Stem Cells (BMSCs) in clinical indications. Not surprisingly, a Cochrane Review investigating the effects of BMSC therapy in the setting of Acute Myocardial Infarction (AMI) reported a marked heterogeneity between trials and concluded that larger study populations and more reliable outcome measures would be needed to evaluate the benefits of BMSC therapy [2].
Originally, the concept of EPCs dates back to a landmark study published in 1997 by Asahara et al. [3], who isolated a ‘putative progenitor endothelial cell’ that was found within the CD34+ mononuclear blood cell fraction. These EPCs were able to differentiate in vitro into an endothelial phenotype and incorporated into sites of neovascularization in vivo. After more than a decade of vigorous research, the exact function of these cells is still uncertain. Therefore, we investigated the phenotype and biological function of a particular EPC subtype that has been extensively studied and was renamed as circulating angiogenic cell (CAC) [4], early EPC [5] or Early Pro-angiogenic Cell (EPC) [6].
However, still considerable debate about the origin and function of EPCs remains and much of this uncertainty is caused by a high degree of confusion about the definition of EPCs. Over the years, different culture protocols have emerged, all claiming to produce EPCs from Bone Marrow (BM) or peripheral blood-derived mononuclear cells. Furthermore, a variety of molecular marker combinations have been utilized for their characterization. Obviously, the ambiguity that surrounds the term ‘EPC’ has not facilitated the understanding of EPC biology.
This lack of phenotypical characterization and the limited knowledge of the underlying EPC biology have, however, not hindered the early use of Bone Marrow-Derived Stem Cells (BMSCs) in clinical indications. Not surprisingly, a Cochrane Review investigating the effects of BMSC therapy in the setting of Acute Myocardial Infarction (AMI) reported a marked heterogeneity between trials and concluded that larger study populations and more reliable outcome measures would be needed to evaluate the benefits of BMSC therapy [2].
Originally, the concept of EPCs dates back to a landmark study published in 1997 by Asahara et al. [3], who isolated a ‘putative progenitor endothelial cell’ that was found within the CD34+ mononuclear blood cell fraction. These EPCs were able to differentiate in vitro into an endothelial phenotype and incorporated into sites of neovascularization in vivo. After more than a decade of vigorous research, the exact function of these cells is still uncertain. Therefore, we investigated the phenotype and biological function of a particular EPC subtype that has been extensively studied and was renamed as circulating angiogenic cell (CAC) [4], early EPC [5] or Early Pro-angiogenic Cell (EPC) [6].
TRANSCRIPTOME ANALYSIS REVEALS THE TRUE NATURE OF IN VITRO CULTURED CAC
In our recently published study we conducted an in silico transcriptome analysis of publicly available microarray data [7]. In this way, we were able to provide abundant evidence that the genetic signature of in vitro cultured CACs (CACiv) closely resembles the transcriptome of regulatory M2 macrophages. We demonstrated a high degree of resemblance between the expression profiles of CACiv and so-called ‘alternatively activated’ M2 macrophages, and more specifically the M2 subtype that is induced by the anti-inflammatory cytokine IL10 and is characterized by the upregulation of CD163 and CCL18 [8]. This subtype was already described as regulatory macrophage by Mosser et al. [9]. Surprisingly and contrary to notion that CAC are some sort of endothelial precursor, our CACiv signature did not show evidence for endothelial differentiation. Except for RNASE1 and GPNMB, we found no sign for the induction of endothelial-specific gene expression in CACs. However, abundant evidence for macrophage differentiation was found in the cytokine profile of CACiv. In-depth analysis of the CACiv signature revealed a multitude of markers typically expressed by M2 macrophages (IL10, CCL17, CCL18, CCL22, scavenger receptors CD163 and MARCO) with a high degree of similarity between CACiv and immune regulatory macrophages associated with tumors based on the expression of a specific cytokinetic fingerprint consisting of CCl2, CCL17, CCL18 and CCL22 [8,10]. This profile was predominantly immune modulatory in nature. Together, these findings do not seem to support the hypothesis that CACiv give rise to cells with an endothelial phenotype, but rather suggest that CACiv are a specific subtype of M2 macrophages with a typical gene expression pattern of which specific genes are also found up regulated in other immune modulatory cell types such as immune-modulating Dendritic Cells (DCs), bone-remodeling osteoclasts and lipid-processing atherosclerotic plaque macrophages. This finding has important consequences for all previous studies making use of the CACiv culture protocol, the conclusions of which, at least those concerning endothelial transdifferentation, now are highly debatable.
Using Ingenuity Pathway Analysis (IPA) software, we disclosed previously unrecognized biological processes in CACiv, such as riboflavin metabolism and liver X receptor (LXR)/retinoid X receptor (RXR) and farnesoid X receptor (FXR)/RXR pathways. Both LXRs and FXRs are cholesterol-sensing nuclear receptors regulating cholesterol homeostasis through upregulation of cholesterol efflux transporters. Moreover, both pathways are capable of down regulating inflammatory gene expression by repression of nuclear factor κB (NFκB) in macrophages, which would endow CACiv with anti-inflammatory properties. Therefore, CACiv therapy might be a convenient treatment strategy to selectively target cholesterol metabolism in atherosclerotic disease and induce reverse plaque remodeling. The finding that riboflavin metabolism is actively upregulated in CACiv, suggests that CACiv could have therapeutic potential under conditions of increased oxidative damage and endothelial dysfunction. Riboflavin or vitamin B2 is the central element of the cofactors Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD). FMN and FAD play important roles in cellular oxidoreductase reactions. They are essential cofactors in cellular energy metabolism and cellular resistance to oxidative damage and in the enzymatic reaction catalysed by Nitric Oxide Synthase (NOS) isoforms.
Together, the biological pathways we found to be upregulated in the CACiv profile, relate to reverse cholesterol transport, immunomodulation, energy metabolism and NO bioavailability. These findings could indicate new treatment options with these in vitro cultured CAC, being in nature immune-modulatory macrophages, with interesting pleiotropic effects in the treatment of atherosclerotic and cardiovascular disease. Alternatively, pathophysiological conditions leading to the impaired in vivofunction of these immune-modulatory macrophages would be able to promoteendothelial dysfunction and atherosclerotic plaque formation and progression. However, future experimental studies are required to confirm these hypotheses.
Using Ingenuity Pathway Analysis (IPA) software, we disclosed previously unrecognized biological processes in CACiv, such as riboflavin metabolism and liver X receptor (LXR)/retinoid X receptor (RXR) and farnesoid X receptor (FXR)/RXR pathways. Both LXRs and FXRs are cholesterol-sensing nuclear receptors regulating cholesterol homeostasis through upregulation of cholesterol efflux transporters. Moreover, both pathways are capable of down regulating inflammatory gene expression by repression of nuclear factor κB (NFκB) in macrophages, which would endow CACiv with anti-inflammatory properties. Therefore, CACiv therapy might be a convenient treatment strategy to selectively target cholesterol metabolism in atherosclerotic disease and induce reverse plaque remodeling. The finding that riboflavin metabolism is actively upregulated in CACiv, suggests that CACiv could have therapeutic potential under conditions of increased oxidative damage and endothelial dysfunction. Riboflavin or vitamin B2 is the central element of the cofactors Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD). FMN and FAD play important roles in cellular oxidoreductase reactions. They are essential cofactors in cellular energy metabolism and cellular resistance to oxidative damage and in the enzymatic reaction catalysed by Nitric Oxide Synthase (NOS) isoforms.
Together, the biological pathways we found to be upregulated in the CACiv profile, relate to reverse cholesterol transport, immunomodulation, energy metabolism and NO bioavailability. These findings could indicate new treatment options with these in vitro cultured CAC, being in nature immune-modulatory macrophages, with interesting pleiotropic effects in the treatment of atherosclerotic and cardiovascular disease. Alternatively, pathophysiological conditions leading to the impaired in vivofunction of these immune-modulatory macrophages would be able to promoteendothelial dysfunction and atherosclerotic plaque formation and progression. However, future experimental studies are required to confirm these hypotheses.
TRANSCRIPTOME ANALYSIS REVEALS CACIV SIGNATURE AS AN IN VIVO RELEVANT GENE EXPRESSION ENTITY
In a third, so far unpublished, part of our microarray analysis study we looked at the spatiotemporal changes in gene expression after myocardial infarction (mouse model) in conjunction with the CACiv gene signature. We correlated the AMI transcriptome data with several gene expression profiles (CACiv, monocytes, macrophages and M2/M1 macrophage gene expression profiles) (Figure 1). Here, we demonstrate that a monocyte-like gene expression profile is predominant in the first hours after MI, shifts towards a mixed monocytic-CAC expression profile by 24-48 hours following AMI, and, interestingly, assumes a CAC-like pattern afterwards; by 8 weeks, the AMI transcriptome had renormalizes to an intermediate profile (one-way ANOVA, p= 4.5x10-7) (Figure 2A).
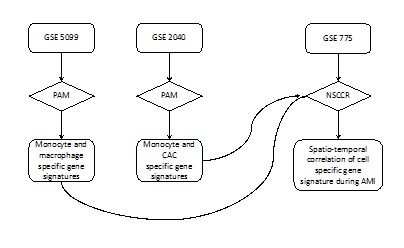
Figure 1: Flowchart of the spatiotemporal correlation of a cell specific gene signature during AMI. GSE: GEO Data Set number; NSCR: Nearest Shrunken Centroid Classification Routine; PAM: Prediction Analysis of Microarrays.
Finally, we correlated the monocyte and macrophage profiles to the AMI transcriptome (Figure 2B, 2C). We observed an influx of a monocyte-associated profile in the first hours after AMI and found evidence of accumulation of macrophage-related gene expression in the subacute phase of AMI. As far as macrophage differentiation is concerned, a temporal down regulation of M1-associated gene expression and a switch towards a predominant M2 expression profile during the remodeling phase of AMI was evident (Figure 2D).
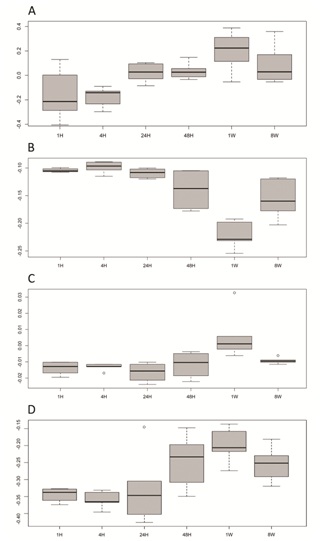
Figure 2: Temporal fluxes of CACiv (A) (p<0.001), monocyte (B) (p<0.001) and macrophage (C) (p=0.002) gene signatures and (D) M2 versus M1 macrophage differentiation (p=0.002) in the AMI transcriptome. The horizontal axis depicts different time points after the induction of AMI (H: hours, W: weeks). On the vertical axis a correlation coefficient (Spearman) indicates for any given time point the degree of correlation between the gene expression during AMI and the cell type specific gene signature. At 1week post AMI, influx of the CAC signature is evident and parallels an M2 versus M1 differentiation of the AMI transcriptome.
Rather than indicating active progenitor cell homing, the influx of the CACiv signature most probably results from the in situ transformation of the inflammatory cell population toward a more regulatory M2 macrophage phenotype that actively promotes immunomodulation, tissue remodeling and angiogenesis in the later stages of the post-myocardial infarction healing phase. This finding validates the CACivtranscriptome as a biologically, in vivo relevant gene expression entity, more specifically in the late post-myocardial infarction healing phase. This could have important clinical implications towards the optimal timing of future stem cell or CACiv therapy in the setting of myocardial infarction and other ischemia-related conditions.
CONCLUSION
In conclusion, our recently published study indicates that CACiv closely resemble regulatory M2c macrophages. CACiv, however, showed surprisingly little evidence of endothelial cell differentiation, excluding the hypothesis that these cells can truly undergo transdifferentation to form endothelial cells. We propose new biological functions by which CACiv, being in nature M2c macrophages, can interfere with cardiovascular pathophysiology, more specifically by immunomodulation, tissue remodeling, enhancement of cholesterol efflux and eNOS-dependent vasculoprotection. Here, we also demonstrated the appearance of a CACiv-like gene expression profile one week after AMI. Rather than indicating active progenitor cell homing we relate this to a local switch from an inflammatory (M1) into a regulatory (M2) macrophage phenotype. Together, our findings provide novel insights into the true nature of CACiv. This not only enables us to reinterpret the conclusions from previous CAC literature, but these new insights could also revive the interest for clinical CAC research by providing new indications for which CAC therapy could be explored in future clinical trials.
FUNDING
This work was supported by the Research Foundation - Flanders (FWO), grant No. G014906. B. Everaert was supported by a Ph.D. fellowship of the Research Foundation – Flanders (FWO).
DISCLOSURES
None
REFERENCES
- Everaert BR, Van Craenenbroeck EM, Hoymans VY, Haine SE, Van Nassauw L, et al. (2010) Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: Focus on Pi3K/AKT/eNOS pathway. Int J Cardiol 144: 350-366.
- Martin-Rendon E, Brunskill S, Doree C, Hyde C, Watt S, et al. (2008) Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev CD006536.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-967.
- Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164-1169.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, et al. (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: 288-293.
- Urbich C, De Souza AI, Rossig L, Yin X, Xing Q, et al. (2011) Proteomic characterization of human early pro-angiogenic cells. J Mol Cell Cardiol 50: 333-336.
- Everaert BR, Van Laere SJ, Lembrechts R, Hoymans VY, Timmermans JP, et al. (2019) Identification of Macrophage Genotype and Key Biological Pathways in Circulating Angiogenic Cell Transcriptome. Stem Cells Int 2019: 9545261.
- Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, et al. (2006) Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211: 487-501.
- Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958-969.
- Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42: 717-727.
Citation: Everaert BR, Laere SJV, Timmermans J-P, Vrints CJ (2019) Using Transcriptome Analysis for Redefining the in vivo Relevance of in vitro Cultured Circulating Angiogenic Cells (CACiv). J Stem Cell Res Dev Ther 5: 015.
Copyright: © 2019 Bert R Everaert, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!
