New Research in the Anatomy of the Myocardium
*Corresponding Author(s):
Trainini JCDepartment Of Cardiac Surgery, Hospital Presidente Perón, Buenos Aires, Argentina
Tel:+54 111540817028,
Email:jctrainini@hotmail.com
Abstract
Introduction and objectives
The torrent guasp hypothesis considers that the myocardium consists of a continuous muscular band beginning at the level of the pulmonary valve and ending at the level of the aortic root limiting both ventricular chambers. The anatomy investigated in this work provides the interpretation for fundamental aspects of left ventricular dynamics.
Methods
Ten hearts of young bovine animals, a ten-year-old human heart obtained from a heart transplant and a heart that corresponds to a human fetus of 23 weeks of gestation were used. After a preparatory action proceeded to the comprehensive examination of its architecture, subsequently the muscle band was unrolled in the helix that conforms it, being the last step the dissection of its muscular bundles.
Results
The muscle band describes two spiral turns with insertion of one of its ends in the path that extends from the pulmonary artery to the orifice of the tricuspid in the so-called lung-tricuspid cord, in front of the aorta; while the other is in the aortic root, in the right and left trigones. This arrangement of the ventricular myocardial band confers ventricular chambers a leading role in cardiac function. The myocardial band is constituted by two so-called basal loop (right and left segments) and apical loop (descending and ascending segments). In all the examined hearts, at the anteroinferior level of the right trigone, a bone core of was found facing downwards to meet the ascending segment muscle fibers of the myocardial band that are inserted on its surface. The microscopic analysis revealed in the hearts bovine and human a trabecular osteochondral matrix. We have called it cardiac fulcrum.
Conclusions
In summary the myocardium consists of three regions: 1) free wall of the right ventricle; 2) free wall of the left ventricle and 3) interventricular septum. The wall of the right ventricle is formed by the right segment of the basal loop whereas that of the left is formed by both the basal loop (left segment) and the descending and ascending segments of the apical loop. The interventricular septum consists of a ventral and a dorsal part. The first portion consists of the left descending segment, the intraseptal band (the final segment of the muscular band) and the anterior septal band. The first two belong to the left ventricle and to the right ventricle the remainder. The posterior region of the septum is composed of the left descending segment (left ventricle dependence) and the posterior septum, corresponding to the right ventricle. The spatial muscle structure adopted by the myocardial muscle band has a double function: a) to limit the ventricular chambers; b) fulfill the suction and ejection action in its role of cardiac pump. The anatomical description of cardiac fulcrum would put an end to the issue of the lack of support of the muscle band to perform its rotational function, as in the cardiac osteo-chondral fulcrum, it finds its insertion point to achieve the necessary lever just as a muscle does with its bone insertion.
Keywords
INTRODUCTION
Classical anatomy of the heart considered that the muscular structure forming the myocardium was homogeneous and compact. Based on this concept, it was described with an external and internal surface enclosing a uniform muscle mass [1]. This structural notion does not explain cardiac mechanics, and hence, it is essential to establish its true internal anatomy. Historically, very little importance was attributed to the spatial arrangement of the fibrous tracts shaping the myocardium [2]. Toward 1980, Francisco Torrest Guasp [3] defined the anatomy of the heart adapted to physiological reality. For this study, the cardiac structure is correlated with an organic machine presenting outstanding characteristics, such as being a propelling suction pump with the size of a human fist and an average weight of 270 grams, which drives 4-6 liters/ minute at a speed of 300 cm/s, consuming only 10 watts, working without interruption during 80 years without maintenance, almost without noise, and no smoke. Its work is equivalent to the daily extraction of 1 ton of water 1 m deep with a mechanical efficiency (work/energy relationship) of 50%, not achieved by man-made machines which only attain 30%. Its efficacy allows ejecting 70% of the left ventricular volume with only 12% shortening of its contractile unit, the sarcomere.
The ventricular myocardium is formed by an assembly of fibers twisting unto themselves similarly to a rope (rope model) (Figures 1 to 3) and flattened laterally as a band, which by producing two spiral turns defines a helix delineating both ventricles and setting their functionality [4,5]. Based on these facts, we find that the spatial organization and the rotational movement of the ventricular fibers in their anatomical arrangement, both at the base and the apical region, correspond to the myocardial muscle band. However, this anatomy which allows unfolding the heart to form a muscle band was not considered valid until its original description.
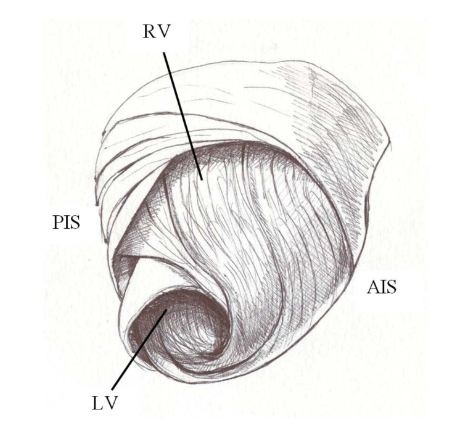 Figure 1: Torrent guasp’s ventricular myocardial band. RD: Right Ventricle; LV: Left Ventricle; PIS: Posterior Interventricular Sulcus; AIS: Anterior Interventricular Sulcus.
Figure 1: Torrent guasp’s ventricular myocardial band. RD: Right Ventricle; LV: Left Ventricle; PIS: Posterior Interventricular Sulcus; AIS: Anterior Interventricular Sulcus.
 Figure 2: Torrent Guasp’s muscle band. (Bovine heart).
Figure 2: Torrent Guasp’s muscle band. (Bovine heart).
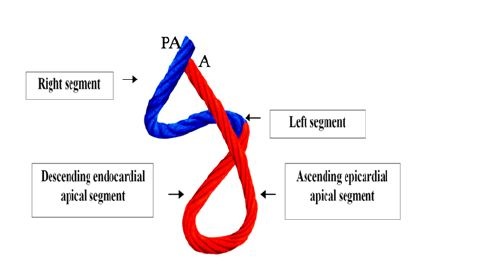 Figure 3: Rope model of the muscle band, showing its different components. In blue: Basal loop. In red: Apical loop. PA: Pulmonary Artery; A: Aorta.
Figure 3: Rope model of the muscle band, showing its different components. In blue: Basal loop. In red: Apical loop. PA: Pulmonary Artery; A: Aorta.
In view of the criticism or indifference to the helical myocardial band proposed by Torrent Guasp, which we believe is due to lack of information and the necessary anatomical technique to perceive it, we can oppose its confirmation through: 1) heart dissection; 2) new imaging procedures obtained with magnetic resonance by diffusion tensor [6-10]; and 3) recent electrophysiological studies performed with three-dimensional electroanatomic mapping [11-15].
Human and bovine hearts were used in this research, the latter for the possibility of greater obtaining facilities, since it has been pointed out that the same plan of cardiac organization exists in mammals. It must be understood that this organ cannot be studied without taking into account that structure and function participate indissolubly united in their complex dynamics. Cardiac mechanics is closely related to the design of its structure constituted by the scaffolding in the wall of the ventricles. Despite the extremely high number of bundles and fascicles that can be described in the myocardium, however, the trajectory and direction obey a predominant axis that constitutes the myocardial band that runs from the pulmonary artery to the aorta and that in spatial composition of helicoid allows for systolic twisting, untwisting-suction and diastolic relaxation.
Andrés Vesalio in his book "De Humanis Corporis Fabrica" (1543), referred to the difficulty in discerning the layers that make up the myocardium. It said verbatim: "Whatever you do dissecting heart meat, whether raw or cooked ... you can barely pull off a portion of a single type of fiber, because they have multiple and distinct directions, especially transverse". Pettigrew (1864) also referred to this situation: "Of the complexity of the disposition I need speak no more than Vesalius, Haller, and De Blainville, all confessed their inability to decipher it" [16].
An explanation for this muscular homogeneity, more apparent than real, implies considering the required functionality in birds and mammals to drive blood at a high speed in a limited time by an organ that must provide two circulations (systemic and pulmonary). Despite these considerations, the myocardium dissection finds a structure with defined planes where the successive and related physiological heart movements of narrowing, shortening-twisting, lengthening-untwisting and widening take place, depending on the propagation of the electrical stimulus along its muscle pathways [15].
The myocardial fibers forming the myocardium cannot be considered as independent entities within a defined space. Despite the intricacy of fiber bundles with polygonal shape, which in addition receive and give off collateral fibers, a predominant trajectory of central fibers is defined with sliding planes, together forming the myocardial muscle band. It should be remembered that the myocardium constitutes a spiral continuum in its fibers responding to a helical pattern in its muscle bundles. This arrangement indicates the need of generating a mechanical work that dissipates little energy. Therefore, the layers of fibers very gradually shift their orientation, with more or less acute angles, to avoid that abrupt changes in the spatial organization waste the necessary work for cardiac function. The range of fibers that is formed reduces the stress between them.
This situation simulating a web of fibers allows the band to act as a continuous chain of transmission by taking the epicardial fibers in an oblique direction, the intermediates a transverse direction and the endocardial ones also oblique but opposite in direction of the epicardial plane. The angle of entry of the endocardial and epicardial planes in relation to the transverse planes is about 60 degrees. The orientation of the fibers determines the function and thus the ejection fraction is 60% when the normal helical fibers contract and falls to 30% if only the transverse fibers are shortened. The latter occurs when the left ventricle is dilated and the fibers lose their oblique orientation with loss of muscular and mechanical efficiency (Figure 4).
 Figure 4: Cross section of both ventricles at the level of atrioventricular valves. It shows the circulatory interlaced layers. (Human heart).
Figure 4: Cross section of both ventricles at the level of atrioventricular valves. It shows the circulatory interlaced layers. (Human heart).
In this way it must be understood that a gradual change of orientation is generated from the surface fibers to the deep fibers. As you progress from the base to the ventricular apex the amount of horizontal fibers decrease relative to the oblique ones demonstrating that the heart is organized in a continuous muscular spiral. Ventricular mechanical activity should be heterogenous during diastole, with a subendocardial-subepicardial relaxation gradient. This happens for systole where the muscular layers of the band at the base show a more pronounced torsion in the subendocardium than in the subepicardium, whereas in the apex the rotation of the subepicardial fibers acquires greater importance.
The helicoid arranged by the ventricular band harmonizes with the disparity of the directions adopted by the fibers. The geometric properties of cardiac fibers are extremely important in the ability to generate, through electrical propagation, forces necessary for their function. This muscular helicoid is composed of muscular layers with skewed directions that allow the muscular band to act as a multiplying belt generating a continuous force as the fibers slide over each other. The anatomical interpretation developed in this work correlates with previous work on cardiac mechanics [11-15], which allows explaining the structure-function correlation.
Beyond this complexity it is necessary to establish the concept of linear and laminar trajectories. Myocardial muscle bundles and bands, deriving from phylogenetic development, essentially form a master axis of precise dynamic requirement. The spatial muscle structure adopted by the myocardial muscle band has a double function: a) to limit the ventricular chambers; b) fulfill the suction and ejection action in its role of cardiac pump.
MATERIAL AND METHODS
Ten hearts of young bovine animals, a ten-year-old human heart obtained from a heart transplant and a heart that corresponds to a human fetus of 23 weeks of gestation were used. The heart to be dissected must be boiled in water with acetic acid (15 cc per liter) for approximately two hours and a half, except that a pressure cooker is used, in which case the time is reduced by half. Prior work before unfolding the muscle band consists in separating the atria from the ventricles in a very simple maneuver that demonstrates the different evolutionary origins between both types of chambers. Then, the aorta and the pulmonary artery are cut three centimeters from their origin, separating the attachment between them; finally, a longitudinal incision is done on the superficial fibers (interband or aberrant fibers) [6,13] extending transversally along the anterior wall of the ventricles. Prior boiling of the anatomical piece allows the easy performance of all these steps. Between the atria and the ventricles there is simply connective tissue on the wall, which allows the chambers to simply be separated, given the denaturation established by the heat.
RESULTS
Left ventricle
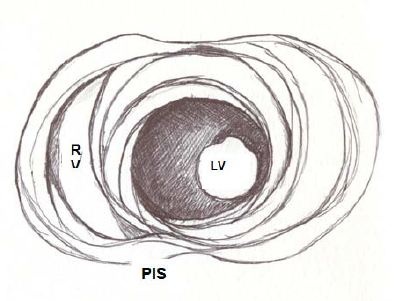 Figure 5: Apical view of the left and right ventricles. RV: Right Ventricle; LV: Left Ventricle; PIS: Posterior interventricular sulcus.
Figure 5: Apical view of the left and right ventricles. RV: Right Ventricle; LV: Left Ventricle; PIS: Posterior interventricular sulcus.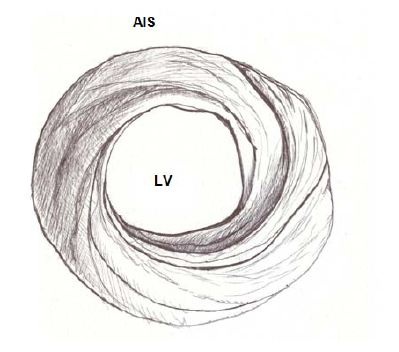 Figure 6: Spiral arrangement of apical muscle layers. AIS: Anterior Interventricular Sulcus; LV: Left Ventricle.
Figure 6: Spiral arrangement of apical muscle layers. AIS: Anterior Interventricular Sulcus; LV: Left Ventricle.In the left ventricular basal half, at the level of its free wall (Figure 7), the fibers are ordered similarly to the apical half. A muscle layer with spiral trajectory extends from the surface to the center arranging paraepicardial and paraendocardial regions from the outside to the inside. At this level their orientation is opposite to that of the apex, following a counter-clockwise trajectory (apical view of the diaphragmatic surface of the heart in anatomical position). This arrangement of the spiraling muscle layer limits a cavity which at the base of the heart is real and not virtual as in the apex.
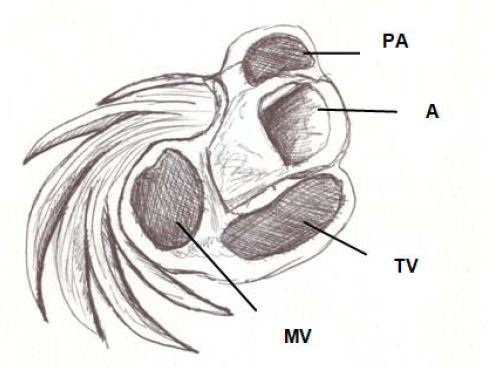 Figure 7: Basal third of the left ventricle, showing the free wall muscle layers. PA: Pulmonary Artery; A: Aorta; TV: Tricuspid Valve; MT: Mitral valve.
Figure 7: Basal third of the left ventricle, showing the free wall muscle layers. PA: Pulmonary Artery; A: Aorta; TV: Tricuspid Valve; MT: Mitral valve.The apex should be considered as a tunnel with a rim in its entire ring, while at the ventricular base this ring has two parts. One part corresponds to the left ventricular free wall and the other to the interventricular septum. In addition, the superficial basal fibers make contact with the fibrous mitral annulus, absent at the apical level. However, the essential functional difference between basal and apical segments is the opposite trajectory of their fibers. This particularity allows a muscular twisting work to achieve the sanguine expulsion from the heart and the distortion to provoke the suction and the filling.
Right ventricle
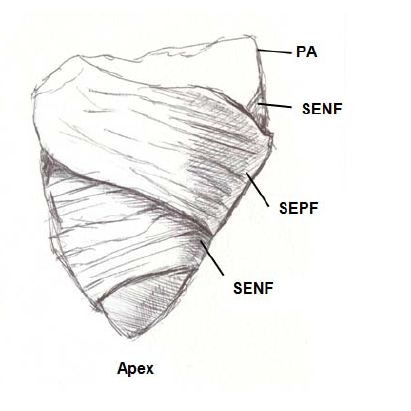 Figure 8: Right ventricular free wall. PA: Pulmonary Artery; SENF: Subendocardial Fibers; SEPF: Subepicardial Fibers.
Figure 8: Right ventricular free wall. PA: Pulmonary Artery; SENF: Subendocardial Fibers; SEPF: Subepicardial Fibers.Three segments can be identified at the basal half of the right ventricle (tricuspid orifice perimeter): free wall, supraventricular crest and interventricular septum. The free wall presents the same general configuration of spiraling fibers that go from subepicardial to subendocardial positions. Similarly to the left ventricle, there is a difference in the rotating sense of the basal fibers with respect to the distal ones. They follow a counterclockwise trajectory at the basal end and a clockwise trajectory at the distal end (apical view of the diaphragmatic surface of the heart in anatomical position).
Segmentation of the ventricular myocardial band
The figure in eight defined by its course outlines two loops: a basal and an apical loop. The basal loop extends from the root of the pulmonary artery to the central twist of the band. On the other hand, the apical loop courses from this twist to the aortic root. Moreover, each loop is formed by two segments. The basal loop consists of the right and left basal segments and the apical loop by the descending and ascending apical segments (Figure 3). In the general configuration, the basal loop embraces the apical loop, so that the right ventricular chamber is more like an open slit in the muscle mass thickness forming both ventricles (Figure 1). The segments are defined by anatomical structures.
Basal loop
Apical loop
These concepts indicate that the right ventricular free wall is formed by one loop (basal) and the left ventricular free wall by both loops (basal and apical). The fundamental fact for cardiac mechanics is that the muscle fibers of the base and apex of the heart move in the opposite direction. This disparity in the commented directions has a correlation between the trajectories reached by the fibers and the helicoid pattern of the muscular band delimiting the ventricles.
Interband fibers
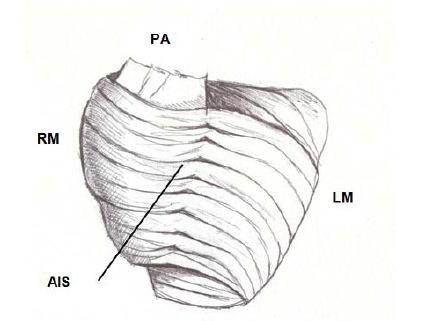 Figure 9: Interband fibers. PA: Pulmonary Artery; RM: Right Margin; LM: Left Margin; AIS: Anterior Interventricular Sulcus.
Figure 9: Interband fibers. PA: Pulmonary Artery; RM: Right Margin; LM: Left Margin; AIS: Anterior Interventricular Sulcus.The possibility was considered that the transmission of the radial impulse between the descending and ascending segments would be through the interbranch or aberrant fibers. In this sense, in the light of what we found in relation to the path of the cardiac stimuli and the simultaneous and opposing activation of the descending and ascending segments (Figure 10), we considered that responsible for this transfer of the stimulus would be the subendocardial fibers of the descending segment in the anterior face of the left ventricle, which pass in depth through the mesocardium crossing perpendicularly with those of the ascending segment (Figure 10) [12].
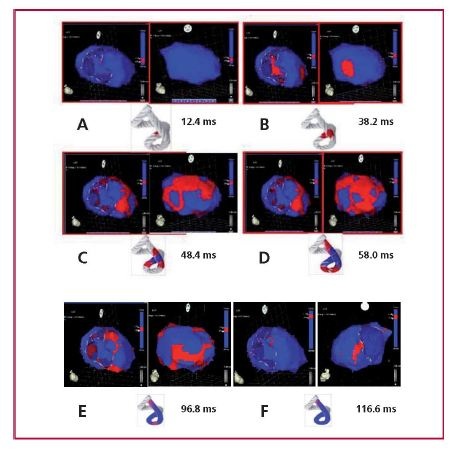 Figure 10: A. Onset of left ventricular activation. The left panel shows the depolarization of the interventricular septum at 12.4 ms, corresponding to the descending band segment. In the right panel, the ventricular epicardium (ascending band segment) has not been activated yet. B. Simultaneous activation of the band segments. The activation progresses in the left ventricular septum through the descending band segment (axial activation) and simultaneously propagates towards the epicardium (radial activation) activating the ascending band segment at 38.2 ms. C. Bidirectional activation of the apex and the ascending band segment. The panel shows the end of septal activation, extending towards the apex, synchronously with the epicardial activation in the same direction. At the same time, the epicardial activation is directed towards the base of the left ventricle (48.4 ms). D. Progression of activation. The panel illustrates the progression of activation in the directions of the previous panel (58 ms). E. Late activation of the ascending band segment. At this moment, corresponding to approximately 60% of QRS duration, subendocardial activation (descending band segment) is already complete. The distal portion of the ascending band segment (epicardial segment) is depolarized later. This phenomenon correlates with its persistent contraction during the initial diastolic phase (96.8 ms). F. Final activation. The right panel shows the very late activation of the distal portion of the ascending band segment after 116.6 ms in a modified left anterior to left posterior-lateral oblique projection (research in patients).
Figure 10: A. Onset of left ventricular activation. The left panel shows the depolarization of the interventricular septum at 12.4 ms, corresponding to the descending band segment. In the right panel, the ventricular epicardium (ascending band segment) has not been activated yet. B. Simultaneous activation of the band segments. The activation progresses in the left ventricular septum through the descending band segment (axial activation) and simultaneously propagates towards the epicardium (radial activation) activating the ascending band segment at 38.2 ms. C. Bidirectional activation of the apex and the ascending band segment. The panel shows the end of septal activation, extending towards the apex, synchronously with the epicardial activation in the same direction. At the same time, the epicardial activation is directed towards the base of the left ventricle (48.4 ms). D. Progression of activation. The panel illustrates the progression of activation in the directions of the previous panel (58 ms). E. Late activation of the ascending band segment. At this moment, corresponding to approximately 60% of QRS duration, subendocardial activation (descending band segment) is already complete. The distal portion of the ascending band segment (epicardial segment) is depolarized later. This phenomenon correlates with its persistent contraction during the initial diastolic phase (96.8 ms). F. Final activation. The right panel shows the very late activation of the distal portion of the ascending band segment after 116.6 ms in a modified left anterior to left posterior-lateral oblique projection (research in patients).DISCUSSION
Cardiac band anatomy
The next step (the most delicate one) consists in entering the aforementioned dihedral angle between the right ventricular and intraseptal fibers. This separation from the right ventricle allows entering a cleavage between the anterior septal band and the intraseptal band (final segment of the myocardial muscle band), at the ventral part of the septum. Then, the dorsal part of the septum is dissected between the posterior septal band and the left descending segment to remove and separate the aorta.
Finally, the trajectories of the descending segment muscular layers are separated in blunt fashion, leaving the trigones attached to the aorta at the opposite end of the muscle band, to the right of the operator, allowing the band to be extended in all its length.
Figure11 shows the sequence of myocardial uncoiling until it becomes a muscle band. The drawing in figure 12 details the different segments comprising it, as well as the insertions of the pulmonary artery and of the aorta.
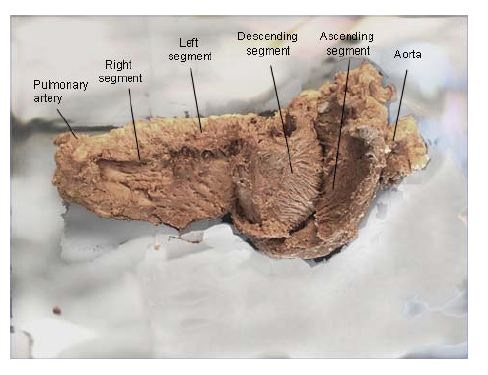 Figure 11: Unfolding the myocardial muscle band. (Bovine heart). In the descending and ascending segments can be seen the fibers spiral according to the helical structure. (Bovine heart).
Figure 11: Unfolding the myocardial muscle band. (Bovine heart). In the descending and ascending segments can be seen the fibers spiral according to the helical structure. (Bovine heart).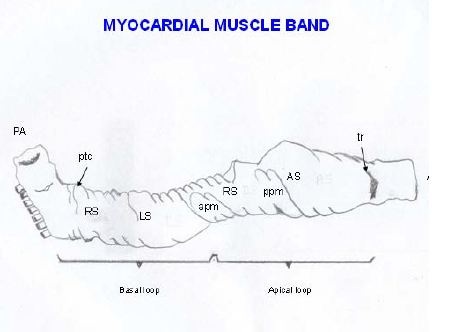 Figure 12: Explanatory diagram of the muscle band segment. A: Aorta; PA: Pulmonary artery; RS: Right Segment; LS: Left Segment; DS: Descending Segment; AS: Ascending Segment; PTC: Pulmo-Tricuspid Cord; TR: Trigones; APM: Anterior Papillary Muscle; PPM: Posterior Papillary Muscle.
Figure 12: Explanatory diagram of the muscle band segment. A: Aorta; PA: Pulmonary artery; RS: Right Segment; LS: Left Segment; DS: Descending Segment; AS: Ascending Segment; PTC: Pulmo-Tricuspid Cord; TR: Trigones; APM: Anterior Papillary Muscle; PPM: Posterior Papillary Muscle.The right ventricular free wall is made up of two groups of muscle fibers (Figure 13) that cross their trajectory in the form of an X. One is the subepicardial right bundle that descends from the posterior interventricular sulcus towards the apex. The second is the subendocardial right bundle that extends from the root of the pulmonary artery to the posterior interventricular sulcus. Between the two bundles other intermediate fibers can be seen which gradually deviate from their direction according to the spiral plan of the myocardium in its functional integrity.
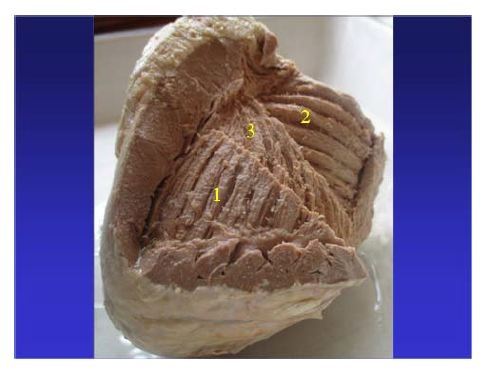 Figure 13: In the free wall of the right ventricle can be observed: 1: subepicardial fibers; 2: subendocardial fibers. In fact this division is simplified for didactic purposes, since between the two contingents of fibers a third one and 3: is evidenced, whose fibers gradually change of direction resembling an open fan. (Human heart).
Figure 13: In the free wall of the right ventricle can be observed: 1: subepicardial fibers; 2: subendocardial fibers. In fact this division is simplified for didactic purposes, since between the two contingents of fibers a third one and 3: is evidenced, whose fibers gradually change of direction resembling an open fan. (Human heart).The transverse subepicardial right bundle defines the tricuspid orifice posteriorly and laterally. It is formed by two bands. The ventral one, called the anterior septal band ends in the pulmo-tricuspid cord. The dorsal one, called the posterior septal band ends at the border of the tricuspid orifice and the free wall of the right ventricle. A small triangle of superior tricuspid base is delimited between both terminal insertions, constituting the membranous septum (Figure 14).
 Figure 14: ASB: Anterior Septal Band; PSB: Posterior Septal Band; MS: Membranous Septum. (Human heart).
Figure 14: ASB: Anterior Septal Band; PSB: Posterior Septal Band; MS: Membranous Septum. (Human heart).If the right subepicardial bundle is resected, the subendocardial right bundle is observed deeper, almost in a vertical position, consisting also of two groups of fibers: a) the pulmonary band and b) the septal band (also called the bridge) (Figure 15). The latter ends in the interventricular septum, forming a raphe, the pulmo-tricuspid cord, in a trajectory between the pulmonary artery and the tricuspid valve.
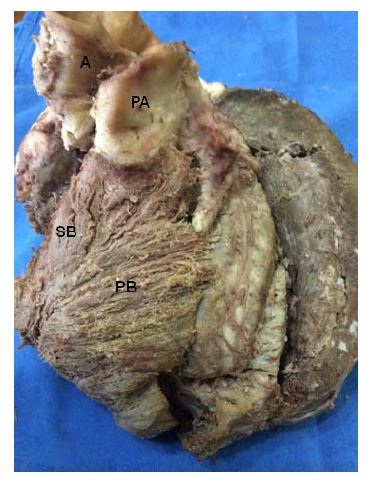 Figure 15: A: Aorta; PA: Pulmonary Artery; SB: Septal Band; BP: Pulmonary Band. (Bovine heart).
Figure 15: A: Aorta; PA: Pulmonary Artery; SB: Septal Band; BP: Pulmonary Band. (Bovine heart).Thus, this defines that the muscle structure of the right ventricle (right band segment), consisting of four groups of fibers (pulmonary, septal, anterior septal and posterior septal), originates at the root of the pulmonary artery, in the pulmo-tricuspid cord and at the border of the tricuspid valve, giving origin to the muscle band of Torrent Guasp.
When addressing the dissection in the external side of the left ventricle there are two groups of fibers (Figure 16) consisting of ascending muscle bundles (belonging to the ascending segment). One group reaches the left trigone, called left trigone band (below the left coronary artery). The second group of muscle fibers follows a direction parallel to the former and when they run in the anterior interventricular sulcus they pass between the right ventricle and the septum to reach the aorta below the origin of the right coronary artery (right trigone), crossing underneath a group of fibers called transversal fibers. This bundle is called right trigone band. If the right ventricle is separated from the left ventricle, the fibers that reach the right trigone are more clearly evident (Figure 17).
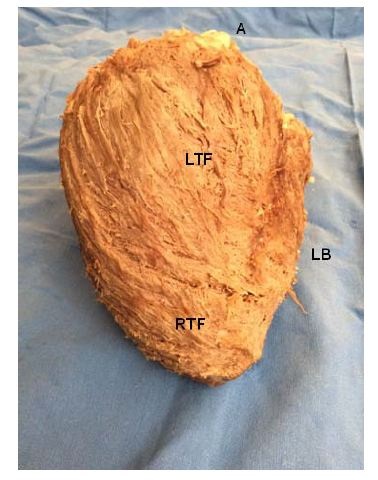 Figure 16: A: Aorta; LB: Left Border; LTF: Left Trigone Fibers; RTF: Right Trigone Fibers. (Bovine heart).
Figure 16: A: Aorta; LB: Left Border; LTF: Left Trigone Fibers; RTF: Right Trigone Fibers. (Bovine heart).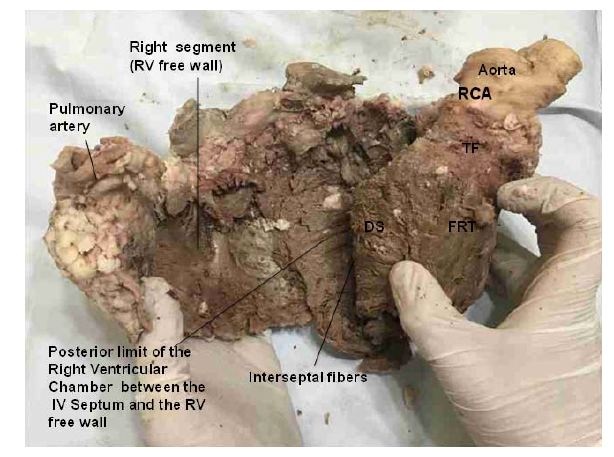 Figure 17: The right ventricle has been separated from the left ventricle along the anterior Interventricular (IV) sulcus. This maneuver allows seeing the Fibers of the Right Trigone (FTR) wich are inserted below the origin of the Right Coronary Artery (RCA). These fibers insert into the aorta passing under the Transverse Fibers (TF). DS: Descending Segment. RV: Right Ventricle. (Bovine heart).
Figure 17: The right ventricle has been separated from the left ventricle along the anterior Interventricular (IV) sulcus. This maneuver allows seeing the Fibers of the Right Trigone (FTR) wich are inserted below the origin of the Right Coronary Artery (RCA). These fibers insert into the aorta passing under the Transverse Fibers (TF). DS: Descending Segment. RV: Right Ventricle. (Bovine heart).Figure 11 shows in detail the muscle fibers of the descending and ascending segments, to achieve the mechanical effect of ventricular torsion through their spiral direction [14]. The subendocardial fibers of the descending segment on the anterior side of the left ventricle pass in depth through the mesocardium crossing perpendicularly with those of the ascending segment. The synthesis of the bands that compose the ventricular mass can be observed in figures 18 and 19; and Table 1.
|
Right Ventricle |
Right subepicardial bundle |
anterior septal band |
|
posterior septal band |
||
|
Right subendocardial bundle |
pulmonary band |
|
|
septal band |
||
|
Left Ventricle |
Left descending segment |
|
|
|
||
|
Right ascending segment |
left trigone band |
|
|
right trigone band |
Table 1: Right and left ventricles.
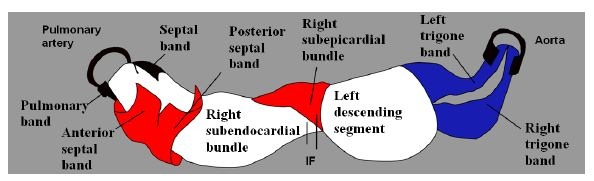 Figure 18: Composition of the myocardial muscle band. IF: Interventricular Fibers.
Figure 18: Composition of the myocardial muscle band. IF: Interventricular Fibers. Figure 19: Cross section of both ventricles. Left ventricle: left; right ventricle: right. (Human heart). 1: Interband fibers; 2: Right descending (subepicardial) bundle; 3: Right ascending (subendocardial) bundle; 4: Anterior septal band; 5: Posterior septal band; 6: Intraseptal band; 7: Descending segment; 8: Ascending segment.
Figure 19: Cross section of both ventricles. Left ventricle: left; right ventricle: right. (Human heart). 1: Interband fibers; 2: Right descending (subepicardial) bundle; 3: Right ascending (subendocardial) bundle; 4: Anterior septal band; 5: Posterior septal band; 6: Intraseptal band; 7: Descending segment; 8: Ascending segment.In figure 20, by removing the septal cusp of the mitral valve located between the two trigones, the right and left trigones are observed in detail. This insertion should be understood as a final point of support for the muscle band at the level of its ascending segment, same as the origin of the band with its right segment (Figures 21 and 22) is attached to the pulmo-tricuspid cord (trajectory between the pulmonary artery and the tricuspid annulus). In contrast, the posterior cusp of the mitral valve should be considered an extension of the ventricular endocardium.
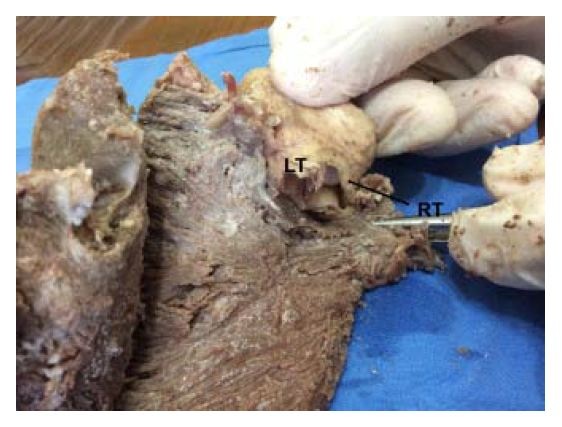 Figure 20: For a more accurate view of the trigones, the septal cusp of the mitral valve lying between them has been removed, exposing the trigones where final end of the muscle band is inserted. LT: Left Trigone. RT: Right Trigone. (Bovine heart).
Figure 20: For a more accurate view of the trigones, the septal cusp of the mitral valve lying between them has been removed, exposing the trigones where final end of the muscle band is inserted. LT: Left Trigone. RT: Right Trigone. (Bovine heart).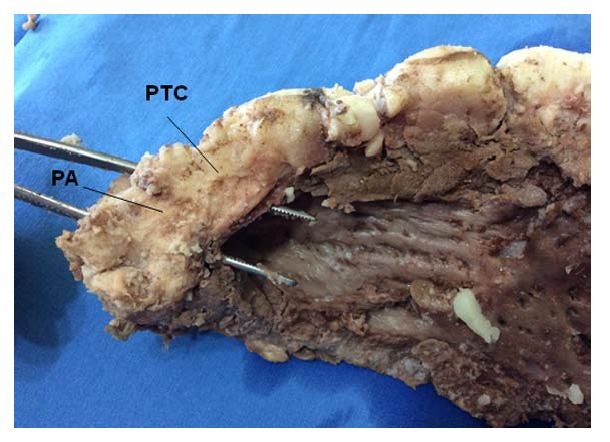 Figure 21: Overview of the Pulmonary Cord (PTC) (origin of the muscle band). PA: Pulmonary Artery. (Bovine heart).
Figure 21: Overview of the Pulmonary Cord (PTC) (origin of the muscle band). PA: Pulmonary Artery. (Bovine heart).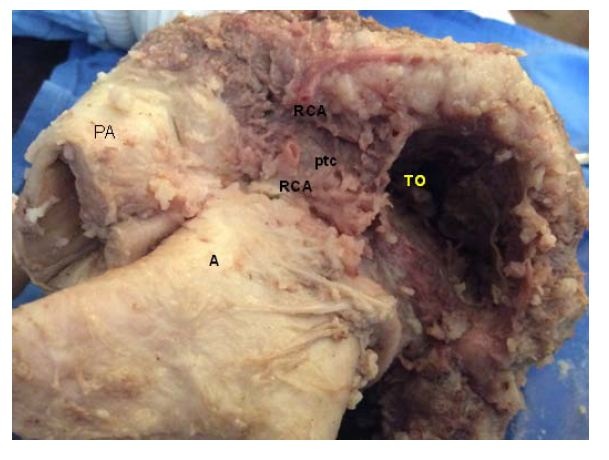 Figure 22: Detail of the Pulmo-Tricuspid Cord (ptc) between the Tricuspid Orifice (TO) and the pulmonary artery (PA), where the myocardial muscle band originates. The Right Coronary Artery (RCA) has been removed to show the cord trajectory. A: Aorta. (Bovine heart).
Figure 22: Detail of the Pulmo-Tricuspid Cord (ptc) between the Tricuspid Orifice (TO) and the pulmonary artery (PA), where the myocardial muscle band originates. The Right Coronary Artery (RCA) has been removed to show the cord trajectory. A: Aorta. (Bovine heart).Torrent Guasp [5] considered that the cardiac muscle lacks a fixed point of support like those depicted by the skeletal muscle system to fulfill its force function. In this sense, he analyzed that the muscle band would act in the same way as the circular muscle of the arteries, and that therefore, it would support itself on the proper contents of the cavity (hemoskeleton). In relation with this point, in all the examined hearts, we have found at the level of the right trigone, a bone core facing downwards to meet the muscle fibers of the ascending segment inserted on its surface. This is located in the vicinity of the tricuspid valve (right), the aorta (posterior) and the pulmo-tricuspid chord (anterior). The hearts of bovine studied with computed tomography, magnetic nuclear resonance and simple radiography showed the osteo-chondroid nucleus found in the dissection, observing the same morphology and analogous size. The microscopic analysis of this structure in bovine and in the ten-year-old human shows a trabecular osteochondral matrix with segmental lines. In the fetus was found pre-chondroid areas. This anatomical description would put an end to the issue of the lack of support of the muscle band to perform its rotational function, as in the cardiac osteo-chondral fulcrum, it finds its insertion point to achieve the necessary lever just as a muscle does with its bone insertion (Figures 23-27). We have called it cardiac fulcrum.
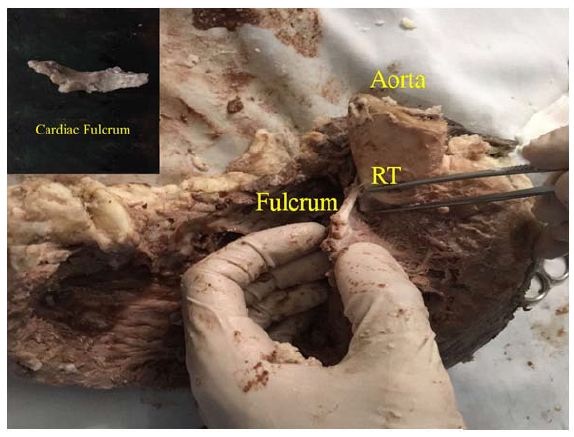 Figure 23: “In situ” cardiac fulcrum below the Right Trigone (RT). The macroscopic observation reveals the cross-linking of the muscle fibers (ascending segments) with the cardiac fulcrum. The inset shows the isolated cardiac fulcrum (bovine heart).
Figure 23: “In situ” cardiac fulcrum below the Right Trigone (RT). The macroscopic observation reveals the cross-linking of the muscle fibers (ascending segments) with the cardiac fulcrum. The inset shows the isolated cardiac fulcrum (bovine heart).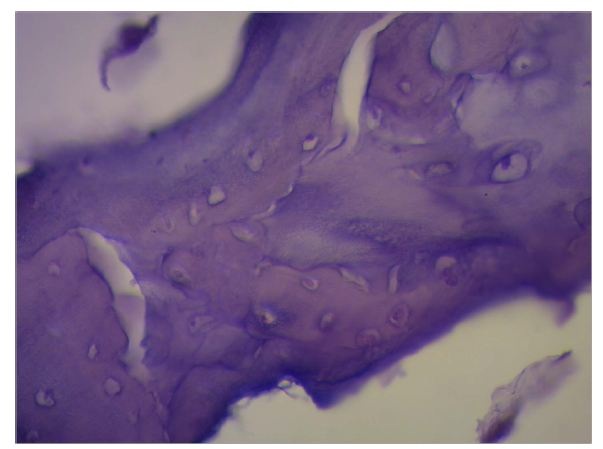 Figure 24: Columns of chondroid tissue and medullary spaces examined with hematoxylin and eosin staining. The structure forms the scaffolding of the trabecular bone tissue similar to the metaphyseal areas of long bones growth. Bone trabeculae are visualized with osteoblasts and segmental lines secondary to bone apposition (bovine heart).
Figure 24: Columns of chondroid tissue and medullary spaces examined with hematoxylin and eosin staining. The structure forms the scaffolding of the trabecular bone tissue similar to the metaphyseal areas of long bones growth. Bone trabeculae are visualized with osteoblasts and segmental lines secondary to bone apposition (bovine heart).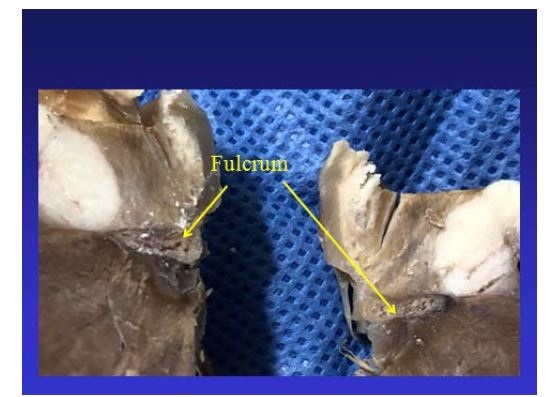 Figure 25: The cardiac fulcrum can be observed in the bovine heart.
Figure 25: The cardiac fulcrum can be observed in the bovine heart.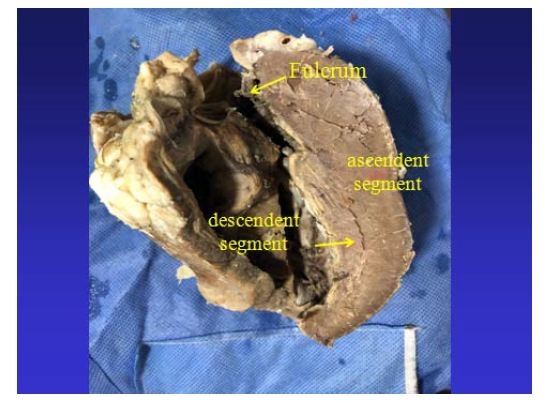 Figure 26: It is observed as the ascending segment surpasses the descending one, the one that runs obliquely to the previous one, and is tied to the cardiac fulcrum (bovine heart).
Figure 26: It is observed as the ascending segment surpasses the descending one, the one that runs obliquely to the previous one, and is tied to the cardiac fulcrum (bovine heart).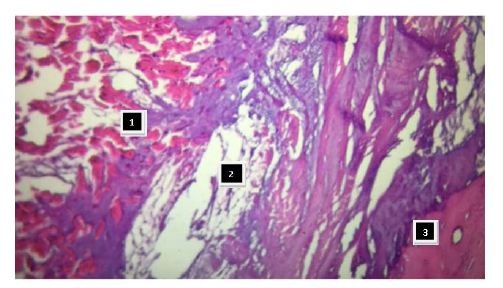 Figure 27: Insertion of the myocardium in the fulcrum. References: 1: myocardial fibers and myxoid stroma; 2: myocardial tapes in a chondroid stroma (insertion); 3: bony cortical tissue of the fulcrum (bovine heart).
Figure 27: Insertion of the myocardium in the fulcrum. References: 1: myocardial fibers and myxoid stroma; 2: myocardial tapes in a chondroid stroma (insertion); 3: bony cortical tissue of the fulcrum (bovine heart).This fixing point could be considered similar to the function exerted by a bearing, preventing the ventricular rotation force, either by torque or twisting effort, to be transferred to the aorta, thus dissipating the energy produced by the helical movement. In relation with this point, the analyzed fixing points become a single piece that supports and allows the band to exercise the fundamental rotation movements of the left ventricle. Let us not forget that the heart is a pendulum within the ribcage without restraints except for those points at the beginning and the end of the muscle band, very close to one another, slightly juxtaposed to the origin of the aorta.
In conclusion, the spatial arrangement and the rotation movement of the ventricular fibers correspond to the architectural plan of the muscle band. Until now, a classic interpretation of blood circulation along the different chambers of the heart has been made, which does not bear correlation with its muscular dynamics. And this, basically, is the circulation engine established by the cardiac band, which also defines with its musculature the limits of the chambers through which the blood flows. This spatial arrangement of the band, mainly at the level of the descending and ascending segments, is what gives the ventricular chambers the fundamental shortening-twisting and lengthening-untwisting movements of cardiac function.
Cardiac apex
The spatial configuration of the double consecutive passage of the descending band situated posteriorly to the ascending band (Figure 3) would allow the apex to turn first to the left during systole (seen from the apex) and then to the right, at the onset of the isovolumic diastolic phase with persisting contraction of the ascending segment. The continuation of descending and ascending bands is a continuum that in this vertex allows the apical loop to act as a bellows that shortens during systole and lengthens during the isovolumic diastolic phase.
As a result of this anatomy-functional process the apex enables the approximation of the base to the tip of the heart during systole (shortening) and its separation during diastole achieving ventricular elongation [12]. This mainly longitudinal functional interplay favors the residual systolic volume (30% of total diastolic volume) in the apical “cul-de-sac”. At this point, we consider that the spatial arrangement of the double consecutive passage of the descending band situated posteriorly to the ascending band allows the non-ejection of part of the cardiac volume at end systole, remaining as residual volume. This remaining fluid acts as a limiting layer for correct suction during the isovolumic diastolic phase.
The apex does not make any measurable movement. It remains practically immobile during the whole cardiac cycle producing only a certain pressure on the chest wall (apex beat). It is the base of the heart which shifts as it descends (reducing ventricular volume) and ascends (increasing ventricular volume).
During systole, the heart undergoes a jet propulsion motion (principle of action and reaction). The apex is the main subordinate region of the retrograde force affecting the ventricular chamber when blood is ejected during systole. Similarly to other body regions with stress overload (ej. JL Petit triangle) it lacks muscle. In addition, it presents precarious irrigation and is submitted to a final pressure in its “cul-de-sac” when the aortic valve closes. This relatively immobile avascular apical area, without interposing muscle, submitted to the maximum effect of left ventricular residual pressure, becomes the place where ventricular wall aneurysms originate in 90% of cases.
Functional aspects of the ventricular myocardial band
Of the three descending band turns relative to the ascending band, the first two successively pass in front and behind the ascending band forming the basal loop. The last step, after the twist of the large band that turns it in the descending segment, it becomes again posterior to the ascending band, with a failed helicoidal arrangement in this space of the apical loop. This spatial anatomical situation of the muscle bands establishes a significant correlation with cardiac function (Figure 3).
The ventricular narrowing movement takes place at the base of the heart, conditioned by the consecutive basal loop right and left segment activation that produces the narrowing phase (systolic contraction). The progression of this phase, in the stimulation process of the descending band (axial), together with the concomitant propagation (radial) towards the ascending band found in our investigations, determines a helical rotating motion with the ensuing shortening of the left ventricular vertical axis (ejection) (Figure 10). The cardiac apex, composed of the subepicardial arrangement of fibers that in their twist become subendocardial, constitute a free apex with a “cul-de-sac” to support the intraventricular pressure produced by the heart impulse during ventricular shortening. The subsequent movement as a result of the ascending band contraction, its straightening and ventricular lengthening therefore determine an active mechanism during isovolumic diastole that maintains the chamber in an isovolumic condition but with intraventricular pressure fall (untwisting and active suction phase) to give place to ventricular filling with atrioventricular opening (expansion phase). These left ventricular shortening (base descent) and lengthening (base ascent) movements correlate with the principle of Newton´s action and reaction principle.
As a result of this anatomo-functional process, the apex (space between the two bands) has in normal conditions the power of annular narrowing (sphincter-like mechanism) to bear the retrograde intraventricular pressure produced by blood ejection.
At the beginning of the isovolumic (active) phase, erroneously considered as belonging to diastole, which instead should be named suction phase, the persistent contraction of the ascending band with left ventricular lengthening generates the fall of intraventricular pressure, sufficient to achieve ventricular suction [17-19]. This pressure fall produced by the persistent contraction of the ascending band during the isovolumic phase with every left ventricular orifice closed, works as a “plunger” mechanism (suction phase) at the moment it starts lengthening. When this pressure is sufficiently low (less than 10 mmHg) and with the ventricle expanded and “unscrewed”, the mitral valve opens and the abrupt flow of blood from the atrium takes place (filling phase).
The contractile impulse of the circulatory tube of the annelids works through a peristalsis mechanism. The propulsion along its length preserves the pattern of axial transmission, but after the twist suffered by the cardiac tube in mammals and birds it adds radial transmission allowing both bands to have a helical motion indispensable to produce the subsequent concatenated twisting and untwisting-suction movements.
It is an arrangement of nature that the descending band passes posterior to the ascending band twice consecutively without wrapping around the latter. In this way it creates a distal cardiac region (apex) favoring the installation of the cardiac residual volume by becoming a zone with less amplitude of helical movement in this game of twisting first to the left during systole (seen from the apex) and then to the right at the beginning of the suction phase with the contraction of the ascending segment. Moreover, it makes the apical loop act as a bellows that shortens during systole and lengthens during the isovolumic phase. This longitudinal motion of apex-base shortening in systole and lengthening during the isovolumic phase is responsible for 75% of left ventricular ejection or suction power. The transverse interplay of basal loop narrowing (systole) and expansion (diastole) contributes to only 25% of cardiac output (Figure 28).
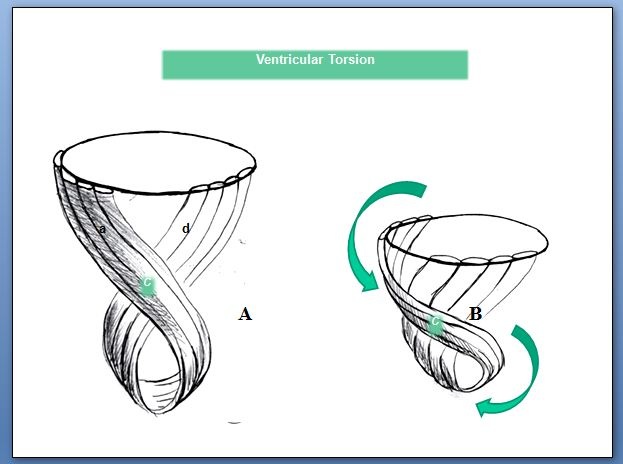 Figure 28: Ventricular twisting. A: Resting ventricular state; B: The subsequent mechanical contraction produces opposed rotation between the ventricular base and apex; C: Band intersection; d: Descending band; a: Ascending band.
Figure 28: Ventricular twisting. A: Resting ventricular state; B: The subsequent mechanical contraction produces opposed rotation between the ventricular base and apex; C: Band intersection; d: Descending band; a: Ascending band.The fact that diastole only employs 15-20% of its period to attain its maximum pressure fall indisputably leads to assume an active mechanical process and not simply passive relaxation. The time employed by diastole to attain its maximum negative pressure (120 ms) is comparable to that used by systole to achieve its maximum ejective pressure (140 ms). Sarcomere architecture in the spatial integration and its contractile framework, as well as its biochemical structure (elastin, titin) implicates elastic properties that add to the active phase. The force generating negative intraventricular pressure through the ascending band activation acts on myocardial elastic properties to achieve the optimal effective sarcomere recoil intime and limit for adequate relaxation. The violation of this limit imposed by the architecture of cardiac muscle will have great impact on heart failure. Neither is systole synonym for contraction nor diastole for relaxation. This denomination is a dialectic that keeps no relation with the real movements that originate ventricular systole, suction and diastole, the three heart stages.
CONCLUSION
Phylogenetic evolution determines an anatomy of the heart that has correspondence with ventricular mechanics. Electrical propagation with three-dimensional electro anatomical mapping researched by us in humans [11-15] explains this correspondence between structure and cardiac function.
Briefly, the myocardium consists of three regions: 1) the free wall of the right ventricle; 2) the free wall of the left ventricle and 3) the interventricular septum. The wall of the right ventricle is formed by the right segment of the basal loop whereas that of the left is formed by both the basal loop (left segment) and the descending and ascending segments of the apical loop; hence, the smaller structural thickness of the right ventricular wall.
The interventricular septum consists of a ventral and a dorsal part. The first portion consists of the left descending segment, the intraseptal band (final segment of the muscle band) and the anterior septal band. The first two belong to the left ventricle and the last one to the right ventricle. The posterior region of the septum is composed of the left descending segment (dependence of the left ventricle) and the posterior septal band, corresponding to the right ventricle.
Historically, the interpretation of blood trajectory along the atrial and ventricular chambers has been made with no correlation between structure and function. The torsion action of the ascending and descending segments, from the evolutionary point of view, allows generating pressure with less energy expenditure and better work effectiveness. Linear propulsion through a circulatory tube would not have this effect. Finally, muscle dynamics is the engine of blood flow. This condition supported by the myocardial anatomy in strict correlation with cardiac function establishes a unit between the ventricles very different from the concept of atrio-ventricular unit which precluded the correct understanding of cardiac physiology. The horizontal arrangement of the atria (venous dependent chambers) became attached to the ventricular muscle component (arterial dependent chambers) where the suction and impulse to produce blood movement resides.
REFERENCES
- Streeter DD, Spotnitz HM, Patel J, Ross J, Sonnenblick EH (1969) Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24: 339-347.
- Aguilar JC (2005) Francisco Torrent Guasp (1931-2005). Rev Esp Cardiol 58: 759-760.
- Torrent Guasp F (1982) Comentarios sobre la forma y la función del corazón. Clin Cardiovasc 1: 85-88.
- Torrent Guasp F, Kocika MJ, Corno A, Masashi K, Cox J, et al., (2004) Systolic ventricular filling. Eur J Cardio-thorac Surg 25: 376-386.
- Torrent Guasp F (1987) Estructura y mecánica del corazón. GRASS. Pg.no:193.
- Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, et al., (2007) Left ventricular form and function revisited: Applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr 20: 539-551.
- Carreras F, Ballester M, Pujadas S, Leta R, Pons-Lladó G (2006) Morphological and functional evidences of the helical heart from non-invasive cardiac imaging. Eur J Cardiothoracic Surg 29: 50-55.
- Cosín-Aguilar J, Hernándiz Martínez A (2013) La disposición de las fibras miocárdicas en una banda condiciona la morfología y la función del corazón. Rev Esp Cardiol 66: 768-770.
- Poveda F, Gil D, Martí E, Andaluz A, Ballester M, et al., (2013) Estudio tractográfico de la anatomía helicoidal del miocardio ventricular mediante resonancia magnética por tensor de difusión. Rev Esp Cardiol 66: 782-790.
- Wu MT, Tseng WYI, Su MYM, Liu ChP, Chiou KR, et al., (2006) Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: Correlation with viability and wall motion. Circulation 114: 1036-1045.
- Trainini JC, Elencwajg B, López Cabanillas N, Herreros J, Lago N, et al., (2015) Electrical propagation in the mechanisms of torsion and suction in a three-phase. Rev Argent Cardiol 83: 416-423.
- Trainini JC, Elencwajg B, López Cabanillas N, Herreros J, Lago N, et al., (2015) Basis of the New Cardiac Mechaniscs: The suction pump lumen, (1st edn) Rev Argent Cardiol, Buenos Aires, Argentina.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lago N, et al., (2015) Propagación eléctrica en los mecanismos de torsión y succión en un corazón de tres tiempos. Rev Argent Cardiol 83: 420-428.
- Buckberg GD, Coghlan HC, Torrent Guasp F (2001) The structure and function of the helical heart its buttress wrapping. VI. Geometrics concepts of heart failure and use for structural correction. Sem Thorac Cardiovasc Surg 13: 386-401.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lago N (2015) Electrophysiological bases of torsión and suction in the continuous cardiac band model. Anat Physiol 5: 1-5.
- Trainini JC (2003) La circulación de la sangre. Biblioteca Médica Aventis. Buenos Aires, Argentina.
- Zarco P (2001) The ventricular rapid filling phase: A muscle relaxation or contraction process? Rev Esp Cardiol 54: 1031-1032.
- Trainini JC, Herreros J, Otero Coto E, Cosin Aguilar J (2011) La «duda clave» de Torrent Guasp: The “key doubt” of Torrent Guasp. Cir Cardiovasc 18: 77-81.
- Trainini JC, Herreros J, Cabo J, Otero Coto E, Cosín Aguilar J (2011) La bomba de succión cardiaca. Aplicación de la banda miocárdica de Torrent Guasp al tratamiento quirúrgico de la insuficiencia cardiaca. Cir Cardiovasc 18: 103-112.
Citation: Trainini JC, Mario B, Alejandro T, Mario W (2019) New Research in the Anatomy of the Myocardium. Trends Anat Physiol 2: 007.
Copyright: © 2019 Trainini JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.