
Buprenorphine/Naloxone and Methadone Opioid Replacement Therapy: A 2-Year Follow-Up Study and Health Economic Analysis
Abstract
Opioid Replacement Therapy (ORT) is the main UK treatment for opiate dependency. Both methadone and buprenorphine-based drugs are licensed for this purpose in the UK with over 25,000 people prescribed in Scotland, mostly receiving methadone. Choice of ORT agent reflects historic guidance that methadone was the ‘first line’ recommendation if both were suitable. Now, evidence suggests that both are equally effective, although concerns regarding a higher risk of methadone overdose have been raised. Many factors, including higher costs and time commitment to dispense buprenorphine-based products, however, may have affected their wider use in the UK. Clinicians require better evidence to inform their clinical decisions. This study considers a cohort of treatment-seeking opiate-dependent individuals in a single health board area in Scotland, prescribed methadone or buprenorphine/naloxone ORT, comparing 2-year retention rates with the costs of treatment delivery and health care utilization.
Methods
We compared 62 patients receiving buprenorphine/naloxone (as Suboxone©) with 175 receiving methadone ORT (Total N=237). The health economic component reports only those for whom a complete dataset was available (n=212). Administrative NHS data was used to assess treatment retention and costs over a two year period. Costs included those associated with ORT delivery as well as broader healthcare utilization.
Results
No statistically significant differences were found with respect to retention rates or healthcare costs though the Cost Effectiveness Plane (CEP) showed considerable uncertainty in these results implying that retention may be greater in the methadone group.
Conclusion
This study suggests that, when combining all treatment delivery and additional healthcare costs, buprenorphine/naloxone is broadly equivalent in cost effectiveness to methadone ORT when delivered in the NHS system. Retention rates over 2 years were also comparable. These data may support the view that buprenorphine/naloxone represents a cost-neutral alternative ORT to that of methadone.
Keywords
INTRODUCTION
Choice of ORT agent
Methadone ORT has been the most common UK treatment for opioid dependence since the 1980s and it is now estimated that some 25,569 people are prescribed this treatment in Scotland [8]. At the turn of the millennium, after successful introduction in mainland Europe, pharmaceutical products based on the opioid agonist/antagonist agent buprenorphine were promoted as an alternative treatment in the UK [11]. Both agents have been associated with a range of positive health, social functioning and criminal justice outcomes. Overall, the accumulated evidence is now felt to support the view that these treatments are equally effective [8,12]. Until recently, however, UK guidance, suggested a hierarchy of use-with methadone preferred if both were clinically indicated [13]. Reflecting the developing evidence-base, the most recent UK treatment guidance, however, has now put both agents on a more equal footing [5].
But these treatments have key clinical differences in terms of patient-experience and delivery. Because of partial antagonist activity, buprenorphine induction presents clinical challenges and potentially more early drop-outs [12]. Additionally, until recently, buprenorphine-based agents were available only as sub-lingual tablets that require longer pharmacy visits, increasing associated costs when supervised dispensing is required. This may be one reason that, while the relative proportions fluctuate, buprenorphine prescribing remains less common than methadone [8]. Also, in urban community pharmacies, where there may be large numbers of patients receiving supervised ORT, the additional time commitment may limit access to ORT if sub-lingual buprenorphine-based products are preferred.
On the other hand, epidemiological studies in the UK have queried increased overdose risk associated with methadone prescribing when compared to buprenorphine [14]. As UK drug deaths are now a major concern, this phenomenon may be one reason that more recent national data has shown an increase in the proportion of buprenorphine prescribing in Scotland [8].
Health economic evaluations
There is little evidence on the difference in wider health care costs between ORT with methadone and any of the standard buprenorphine-based treatments (generic, Subutex© or Suboxone©). As more varied formulations of buprenorphine-such as depot preparations - have become available in the UK recently [20], more valid evidence is required to help direct clinical care delivery, ensuring that patients receive the most appropriate ORT.
Aims and objectives
1. Assess treatment and wider health care costs associated with methadone and buprenorphine/naloxone ORT;
2. Assess the relative rates of retention in treatment at two years;
3. Compare the cost-effectiveness of these drugs.
METHODS
Sample
Data acquisition process
Datasets accessed
Cost estimation
|
Treatment |
Unit Cost |
Reference |
|
|
Methadone |
Buprenorphine/Naloxone |
||
|
Dispensing fee |
£2.15 |
£2.15 |
(ISD Scotland 2012/13) |
|
Instalment fee |
£1.79 |
- |
Recorded by study |
|
Supervision fee |
£1.40 |
£3.40 |
Recorded by study |
|
Drug service visit |
£60.00 |
£60.00 |
(ISD Scotland 2012/13) |
In order to cost the treatment, two datasets were combined: The NHS Fife Addiction (FA) and the Community Prescribing (CP) data. Start dates were chosen for each cohort member as the closest date in either file to October 2011. The NHS Fife Addiction (FA) dataset provided prescription cycles (28 days) as well as a record indicating when pharmacist supervision was required. However, as this local dataset was incomplete, the national Community Prescribing (CP) data was used to address any gaps. This CP dataset contained no indication of prescription cycles or supervision, and did not have accurate prescribing initiation dates (as in these records these were rounded up or down to the nearest month), but was used only to fill gaps in the FA data. Assumptions were made as to the presence of duplicated data based on the dates and doses recorded. In cases where it was not possible to tell if prescriptions were duplicates or not, the CP data was kept in the final combined dataset. Although this may lead to overestimating of the treatment costs overall, it kept the calculation method consistent across the two drug groups and ensured no underestimation of costs. The FA dataset was also found to contain some overlapping dates on prescription records. When identified, these records were assessed by two clinicians in the study team who advised these anomalies were likely due to changes in doses mid 28 day cycle (reflecting more urgent dose adjustments during this phase of treatment). The cycles were adjusted accordingly to reflect shorter cycles on different doses of the ORT drug.
Treatment delivery costs
Regarding service attendances, the FAS standard was that an ORT patient would be seen at clinic on a monthly basis-whatever drug was prescribed. Additional visits may be organised at times of higher risk, when contact may be escalated. We therefore costed one monthly visit with a nurse as the minimum that would have occurred. This was costed using the average nurse-led outpatient clinic visit unit cost (£60), since no specific drug service costs were available.
Healthcare resource utilization
Out-patient clinic attendances
Hospital attendances
Outcome measure
Cost-Effectiveness Analysis (CEA)
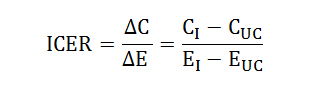
Handling uncertainty
Sensitivity analysis
Information and research governance
RESULTS
Representativeness of sample
Exclusions
The baseline characteristics following this exercise are shown in table 2.
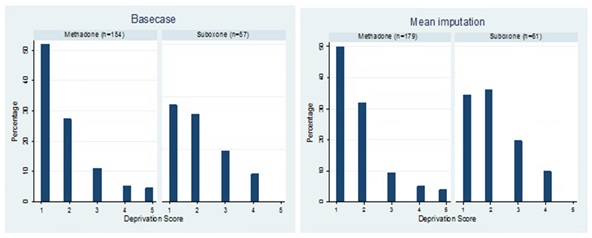
Comparison of baseline characteristics following these exclusions showed that buprenorphine/naloxone and methadone groups did not differ by age (t-test: p=0.522), gender (χ2test: p=0.474) in the basecase, nor in the mean imputation analysis (Table 2). Figure 1 reports the scores at baseline for both groups and analyses using the Scottish Index of Multiple Deprivation [27], which is divided into quintiles from 1 (most deprived) to 5 (least deprived). There were no statistically significant differences between groups in the basecase analysis (ANOVA: p=0.224) nor the mean imputation analysis (ANOVA: p=0.130).
|
Basecase |
Mean Imputation |
|||||
|
Methadone (n=154) (SD) |
Buprenorphine/ Naloxone (n=57) (SD) |
p-value |
Methadone (n=179) (SD) |
Buprenorphine/ Naloxone (n=61) (SD) |
p-value |
|
|
Age |
36 (8) |
37 (10) |
0.5217 |
36 (8) |
37 (10) |
0.5694 |
|
% Female |
35% |
30% |
0.474 |
34% |
31% |
0.675 |
Outcome-retention in treatment
Treatment delivery costs
|
Basecase |
Mean Imputation |
|||
|
Methadone (n=154) (SD) |
Buprenorphine/ Naloxone |
Methadone (n=179) (SD) |
Buprenorphine/ Naloxone |
|
|
Pharmaceuticals |
£425 (£324) |
£2381 (£1500) |
£426 (£324) |
£2382 (£1501) |
|
Dispensing |
£1337 (£348) |
£1373 (£273) |
£1337 (£352) |
£1348 (£298) |
|
Instalments |
£1113 (£322) |
£52 (£207) |
£1106 (£331) |
£49 (£200) |
|
Supervisions |
£405 (£308) |
£356 (£554) |
£427 (£331) |
£401 (£596) |
|
Visits |
£1337 (£361) |
£1327 (£298) |
£1343 (£353) |
£1327 (£298) |
|
Total |
£4612 (£1212) |
£5552 (£1819) |
£4639 (£1256) |
£5507 (£1848) |
|
Adjusted difference (95% CI) |
£918 (£406 to £1430) |
£854 (£361 to £1347) |
||
|
p<0.001 |
p=0.001 |
|||
|
Unadjusted difference (95% CI) |
£938 (£432 to £1446) |
£868 (£372 to £1364) |
||
|
p<0.001 |
P=0.001 |
|||
The adjusted difference in mean total treatment costs was £918 greater for buprenorphine/naloxone, driven by the mean cost of the drug formulation (Suboxone©). This difference was statistically significant. The mean imputation analysis results showed buprenorphine/naloxone treatment costs to be £854 greater than for those with methadone. Again this difference was statistically significant (p < 0.001).
Wider health care costs
|
Basecase |
Mean Imputation |
|||
|
Methadone (n=154) (SD) |
Buprenorphine/ Naloxone |
Methadone (n=179) (SD) |
Buprenorphine/ Naloxone |
|
|
Outpatient cost |
£368 (£611) |
£397 (£825) |
£356 (£587) |
£397 (£801) |
|
A&E cost |
£381 (£559) |
£161 (£350) |
£383 (£547) |
£160 (£468) |
|
Inpatient cost |
£1330 (£4476) |
£466 (£1650) |
£1652 (£6991) |
£445 (£1620) |
|
Psychiatric hospital cost |
£547 (£3173) |
£201 (£1410) |
£917 (£5994) |
£202 (£1365) |
|
Community prescribing cost |
£513 (£725) |
£728 (£1261) |
£501 (£695) |
£730 (£1248) |
|
Total |
£3147 (£5766) |
£1956 (£3103) |
£3811 (£9934) |
£1935 (£3008) |
|
*Adjusted Difference (95% CI) |
-£1258 (-£2502 to -£13) |
-£1207 (-£3039 to £625) |
||
|
p-value=0.048 |
p-value=0.197 |
|||
|
**Unadjusted Difference (95% CI) |
-£1383 (-£2617 to -£148) |
-£1570 (-£2869 to -£272) |
||
|
p-value=0.028 |
p-value=0.018 |
|||
Total costs
|
Basecase |
Mean Imputation |
|||
|
Methadone (n=154) (SD) |
Buprenorphine/ Naloxone |
Methadone (n=179) (SD) |
Buprenorphine/ Naloxone |
|
|
Treatment |
£4612 (£1212) |
£5552 (£1819) |
£4639 (£1256) |
£5507 (£1848) |
|
Healthcare |
£3147 (£5767) |
£1956 (£3103) |
£3811 (£9934) |
£1935 (£3008) |
|
Total |
£7760 (£5875) |
£7508 (£3631) |
£8450 (£9828) |
£7443 (£3579) |
|
*Adjusted Difference |
£-121 (-£1341 to £1098) |
-£697 (-£1957 to £581) |
||
|
p=0.846 |
p=0.285 |
|||
|
**Unadjusted Difference |
£-257 (-£1580 to £1067) |
-£894 (-£2429 to £641) |
||
|
p=0.704 |
p=0.254 |
|||
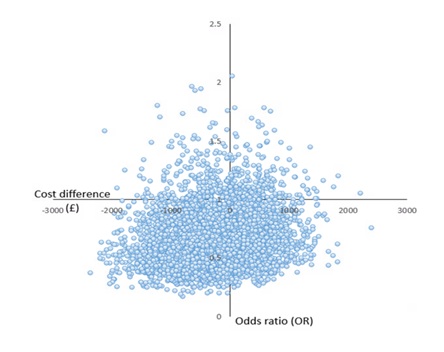
Cost-effectiveness analysis and uncertainty
Sensitivity analysis
Results are shown in table 6. Excluding crossovers had little effect on the headline results, with slightly higher cost difference -£434 in favor of Suboxone© (95% CI=-£2134 to £1265), and marginally less difference in retention, with those in the Methadone group 1.49 times more likely to be retained. Excluding healthcare cost outliers at baseline (those greater than three times the standard deviation of the whole group) and the four deaths (all in the methadone group) also had little effect. All sensitivity analyses gave non-statistically significant results, highlighting further the uncertainty surrounding these findings.
|
Adjusted Difference in Retention (95% CI) |
p-value |
Adjusted Difference in Cost (95% CI) |
p-value |
|
|
Excluding crossovers |
0.67 (0.34 to 1.34) |
0.26 |
-£434 (-£2134 to £1265) |
0.616 |
|
Excluding outliers at baseline |
0.72 (0.37 to 1.375) |
0.316 |
-£327 (-£1938 to £285) |
0.691 |
|
Excluding deaths |
0.59 (0.30 to 1.12) |
0.105 |
-£40 (-£1382 to £1302) |
0.624 |
DISCUSSION
Using informatics techniques, we have compared patients on methadone and buprenorphine/naloxone (as Suboxone©) ORT over two years and have assessed the ability of both agents to retain people in treatment. We have assessed the cost associated with delivering this treatment and have also considered broader healthcare costs incurred, reflecting these patients’ use of NHS services. A Health Economic Analysis has been undertaken to determine which ORT agent is most cost effective at delivering retention in treatment.
We found no statistically significant difference in cost effectiveness between methadone and buprenorphine/naloxone ORT with regard to retention in treatment at 2 years. There was, however, considerable uncertainty in these results with the Cost Effectiveness Plane (CEP) showing most points below the line.
The biggest driver of cost for buprenorphine/naloxone was the total treatment cost, which, at £918 (95% CI=£406 to £1430) was more costly than methadone and statistically significant (p<0.001). This finding is unsurprising due to the much higher pharmaceutical costs of the form of drug used in the study region during the study period-Suboxone©. The greatest driver of cost for methadone, however, was that of inpatient hospital care, reflecting greater healthcare utilisation- potentially reflecting less reduction in harms-in this group.
Strengths and weaknesses of the study
One weakness is the potential bias in the creation of the original audit sample. The statistically significant difference between the groups at baseline in terms of deprivation status and living conditions suggests they may reflect patient groups whose dependency is at a different stage of development associated with different degrees of harm. The buprenorphine/naloxone sample consisted of all patients prescribed the drug at the index date. The methadone group, however, was opportunistically recruited over a similar time period from a larger population on methadone ORT in the same Scottish region. The demographic analysis showed the methadone group to be living in more urban environments and more deprived areas at baseline. This feature alone could have driven some of the differences in “cost” as the more deprived group may be more likely to require to access healthcare [28]. The groups may also have already been “selected” and matched to each treatment in the fife service-as many services report using buprenorphine-based ORT in patients who are more stable or are felt to have more recovery potential. We have not been able to determine whether this difference may further skew our results and conclusions. Finally, the study did not use a randomised sample and we cannot therefore remove the potential effects of confounders.
As reflected in the methods section, many assumptions were made due to the quality and complexity of the available, routinely-collected, administrative data used. Regarding the costs, the greatest uncertainty lay with the combining of the two prescribing datasets. It would be beneficial in future to improve the quality assurance of the NHS Fife Addiction dataset, so that this one dataset-with richer data - can be used for research purposes. The costs we used in the analysis included the use of administrative data (the Scottish Morbidity Record or SMR). These data are not collected for research purposes and, though they contain valuable information, recorded by clinicians and administrators, they inevitably bring further uncertainty. Non-NHS costs – such as social care or criminal justice costs - were not included within this analysis as the data available were not sufficient to give accurate estimates of these wider societal costs, all of which are affected by substance use. This information would have strengthened the analysis considerably.
Regarding outcomes, it has been argued that retention is a valuable “proxy” measure of clinical outcomes – as it is strongly associated with the clinical goals of reduced risk-taking, less drug use and improved health and social functioning. As such, retention can be a helpful indicator of successful treatment and has been reported as such in numerous previous publications. However, in this study, there were no data available to examine those who were not retained. Instead expert clinical advice was sought and it was felt most prudent to assume that any participants leaving treatment before the two year end point should be classed as “not retained”. One participant who stopped ORT but started naltrexone before leaving the treatment service had objectively achieved abstinence so was classed as “retained”.
The overall uncertainty around our findings is a concern. These issues would benefit from further investigation, such as through a well-designed prospective Randomised Controlled Trial (RCT) using a more robust outcome measure such as Quality Adjusted Life Years (QALYs) [29]. QALYs is a generic health outcome measure that can be compared across all intervention types and is recommended in the UK by NICE and SMC for economic evaluations aiming to advice on cost-effectiveness. An RCT may also help to explore further the wider healthcare costs associated with ORT in a more robust fashion.
This study has shown how real life data extraction can be used in an economic evaluation of treatment for opioid dependency and that buprenorphine-based ORT remains a viable alternative to methadone in a UK community treatment setting. In the context of increasing drug death in the UK, more well-designed prospective studies, using robust data, are required to aid clinical decision-making and improve patient choice.
ACKNOWLEDGEMENT
This project could not have been completed without the support of NHS Fife Addiction Services; Health Informatics Centre (HIC), Farr Institute, Dundee-Jim Galloway and Mikhail Ghattas; research assistants Dr Nicola Torrance and Dr Cassie Higgins.
DECLARATIONS OF INTEREST
REFERENCES
- EMCDDA (2012) Annual Report on the State of the Drugs Problem in Europe 2012. EMCDDA, Lisbon, Portugal.
- ISD Scotland (2014) Estimating the National and Local Prevalence of Problem Drug Use in Scotland 2012/13. ISD Scotland, Edinburgh, UK.
- Office for National Statistics (2016) Deaths related to drug poisoning in England and Wales: 2015 registrations. Office for National Statistics, London, UK.
- National Records of Scotland (2016) Drug-related deaths in Scotland in 2015. National Records of Scotland, Edinburgh, UK.
- Department of Health and Social Care (2017) Drug misuse and dependence: UK guidelines on clinical management. Department of Health and Social Care, London, UK.
- United Nations office on Drugs and Crime (1995) The Social Impact of drug Abuse. United Nations office on Drugs and Crime, Vienna, Austria.
- Public Health England (2018) Alcohol and drugs prevention, treatment and recovery: Why invest? Public Health England, London, UK.
- ScotPHO Public Health Information for Scotland (2016) Drug misuse: Treatment for drug misuse. ScotPHO Public Health Information for Scotland, Edinburgh, UK.
- Advisory Council on the Misuse of Drugs (2015) How can opioid substitution therapy (and drug treatment and recovery systems) be optimised to optimise recovery outcomes for service users? Advisory Council on the Misuse of Drugs, London, UK.
- White WL (2010) Recovery-Oriented Methadone Maintenance. Jointly published by the Great Lakes Addiction Technology Transfer Center, the Philadelphia Department of Behavioral Health and Mental Retardation Services, and the Northeast Addiction Technology Transfer Center, in part under a cooperative agreement with the Substance Abuse and Mental Health Services Administration’s (SAMHSA) Center for Substance Abuse Treatment (CSAT), Chicago, USA.
- SUBUTEX et génériques.
- Mattick RP, Breen C, Kimber J, Davoli M (2014) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 3.
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, et al. (2007) Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. Health Technol Assess 11: 1-171.
- Marteau D, McDonald R, Patel K (2015) The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open 5: 007629.
- Mattick RP, Ali R, White JM, O'Brien S, Wolk S, et al. (2003) Buprenorphine-Naloxone versus methadone maintenance therapy: A randomised double-blind trial with 405 opioid-dependent patients Addiction 98: 441-52.
- Canadian Agency for Drugs and Technologies in Health (2013) Suboxone versus methadone for the treatment of opioid dependence: A review of the clinical and cost-effectiveness. Canada.
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, et al. (2014) Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction 109: 79-87.
- Gouveia M, Sousa R, Costa J, Borges M (2015) Economic evaluation of Suboxone® for substitution treatment of opioid drug dependence in Portugal. Heroin Addiction & Related Clinical Problems 17: 43-50.
- Maas J, Barton G, Maskrey V, Pinto H, Holland R (2013) Economic evaluation: A comparison of methadone versus buprenorphine for opiate substitution treatment. Drug Alcohol Depend 133: 494-501.
- Public Health england (2018) National intelligence network on drug health harms briefing: July 2018. Public Health England, London, UK.
- Scottish Medicines Council (2016) Working with SMC-A Guide for Manufacturers. Scottish Medicines Council, Glasgow, Scotland.
- Curtis L, Burns A (2015) Unit Costs of Health and Social Care 2015. Personal Social Services Research Unit (PSSRU), Canterbury, UK.
- ISD Scotland (2013) Prescription Cost Analysis for financial year 2012/13. ISD Scotland, Edinburgh, UK.
- ISD Scotland (2015) IRF- NHS Scotland expenditure-Hospital inpatient and day cases mapping sections, 2013/14. ISD Scotland, Edinburgh, UK.
- NHS Scotland (2015) Methods and Sources-NHS and Social Care. NHS Scotland, Edinburgh, UK.
- Severens JL, De Boo TM, Konst EM (1999) Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 15: 608-614.
- ISD Scotland (2016) The Scottish Index of Multiple Deprivation (SIMD). ISD Scotland, Edinburgh, UK.
- MacLeod, M, Graham, E, Johnston, M, Dibben, C, Morgan I (1999) How does relative deprivation affect health? Health Variations 3: 12-13.
- Richardson G, Manca A (2004) Calculation of quality adjusted life years in the published literature: A review of methodology and transparency. Health Econ 13: 1203-1210.
Citation: Kidd B, Renwick C, Parrott S, Matthews K, Baldacchino A (2019) Buprenorphine/Naloxone and Methadone Opioid Replacement Therapy: A 2-Year Follow-Up Study and Health Economic Analysis. J Addict Addictv Disord 6: 24.
Copyright: © 2019 Brian Kidd, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

