
Human Mesenchymal Stromal Cells-Derived Conditioned Medium Based Formulation for Advanced Skin Care: in vitro and in vivo Evaluation
*Corresponding Author(s):
Raviraja N SeetharamStempeutics Research Pvt Ltd, Karnataka, India
Tel:+91 08202570367,
Fax:+91 08202570368
Email:raviraja.ns@stempeutics.com
Abstract
Keywords
Anti-aging, Conditioned medium; Cytokines; Growth factors; Mesenchymal stromal cells; UVB radiation
INTRODUCTION
Chronic exposure of human skin to solar UV radiation is known to damage the structure and function of the skin [6,7]. These changes are collectively known as photoaging, which is characterized by wrinkles, laxity, roughness and irregular pigmentation [8]. Ultraviolet radiation, especially UVB (280-320 nm) from sunlight, is one of the major environmental hazards to induce skin damage [7]. UV exposure can cause edema, erythema, hyper pigmentation, hyperplasia, photoaging, inflammation, DNA damage and cancer in the skin [8]. Wrinkles are an outward sign of cutaneous aging appearing preferentially on Ultraviolet B (UVB) exposed areas.
Products and therapies that can reduce (or) block (or) reverse this aging process are very important materialsfor research & product development. Mesenchymal stem cells are multipotent cells, which are used in many fields of regenerative medicine. The stem cells of mesenchymal origin are widely reported to secrete a wide range of growth factors which potentially have several benefits for the human skin [9]. The secreted factors alone can be used for many tissues or organ repair due to its various pharmacologically useful growth factors and cytokines. These pharmacologically active factors, known as secretome, can be separated from the culture medium which is used to support the stem cells growth in culture. The used medium with these secreted growth factors and cytokines is referred to as the Conditioned Medium (CM) [9].
In recent years, the stem cell-derived CM is getting more attention in the field of regenerative medicine which is attributed to the various pharmacological active factors present in the CM. The CM enriched in growth factors/cytokines is not only used in regenerative medicine [9], but also its potential role as a cosmetic ingredient is being explored. Numerous studies have investigated the stem cell derived CM as a skin moisturizing and anti-aging agent. Kwon et al., have reported that the conditioned medium from human bone marrow?derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles [11]. Liu et al., showed that umbilical cord mesenchymal stem cells CM protects against photo aging induced by UVA and UVB radiation and is a promising candidate for skin anti-photo aging treatments [12]. Sohn et al., demonstrated the anti-aging properties of CM of epidermal progenitor cells derived from mesenchymal stem cells [13]. Moreover, CM derived from various stem cells have been used in anti-aging formulations and hair growth promoting cosmetic formulations [13-15]. Therefore, this study aimed to develop the BM-MSC derived CM based formulation and elucidate its UV?protective effects. Comprehensive analysis of mesenchymal stromal cells derived CM was carried out and it was formulated as a topical formulation and tested through validated in vitro and in vitro models for its potential use in the area of cosmetics. Growth factors and cytokines present in CM were enriched by concentrating the CM through tangential flow filtration by tenfold (10X CM) to get a better biological effect of the CM.
MATERIALS AND METHODS
Mesenchymal stem cell culture and conditioned media preparation
Particle size analysis
HPLC qualitative analysis of conditioned medium
Analysis of growth factors
Pre-formulation studies of concentrated CM
Formulation development
Migration assay
Cyclobutane Pyrimidine Dimers (CPD) assay
In vivo evaluation of the formulation in nude mice model
Statistical analysis
RESULTS AND DISCUSSION
Particle size analysis of concentrated conditioned medium
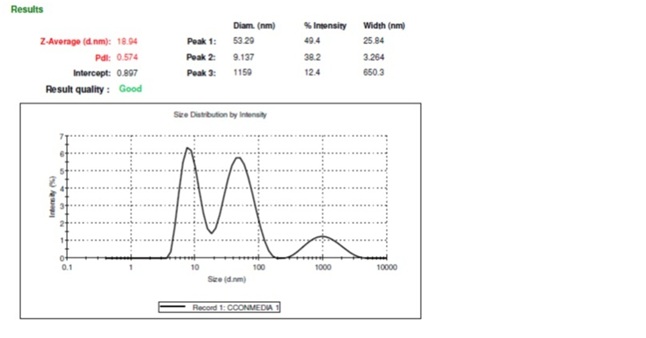 Figure 1: Particle size analysis of concentrated conditioned medium as measured by dynamic light scattering method. The polydispersity index (PDI) (0.574) showed that the CM contains uniform-sized dispersed particles which are in the nano-size range (18.94 nm).
Figure 1: Particle size analysis of concentrated conditioned medium as measured by dynamic light scattering method. The polydispersity index (PDI) (0.574) showed that the CM contains uniform-sized dispersed particles which are in the nano-size range (18.94 nm).Qualitative analysis of growth factors present in the concentrated conditioned medium
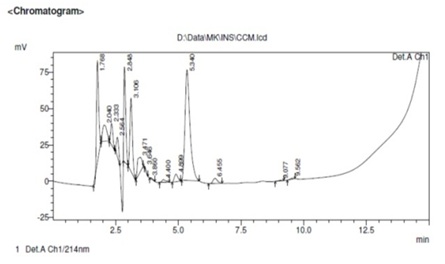 Figure 2: Qualitative analysis of growth factors present in concentrated conditioned medium by HPLC method. The analysis showed numerous peaks in the chromatogram confirming the presence of many dispersed proteins in the CM.
Figure 2: Qualitative analysis of growth factors present in concentrated conditioned medium by HPLC method. The analysis showed numerous peaks in the chromatogram confirming the presence of many dispersed proteins in the CM.Analysis of growth factors
The analysis provides evidence that the bone marrow mesenchymal stem cell-derivedCM contains many cells secreted cytokines and growth factors dispersed in the solutions. The cell signaling factors like FGF7, IL6, IL11, TGF-β, and PDGF are important in wound healing process. The PDGF, VEGF, HGF, M-CSF etc. are important in hair growth. The Angiopoietin, PDGF, IL-6, TGF-β, M-CSF, VEGF, Laminin etc. are important for skin health. Due to the presence of various cytokines and growth factors in the CM, the CM might be an important bioactive material in the area of regenerative medicine [10].
Pre-formulation studies of concentrated CM
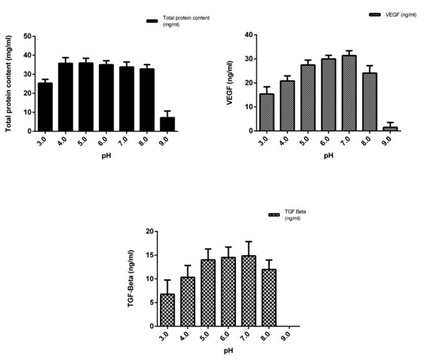 Figure 3: Preformulation studies of the concentrated conditioned medium at different pH conditions.
Figure 3: Preformulation studies of the concentrated conditioned medium at different pH conditions.3a) Total protein content.
3b) VEGF content.
3c) TGF-β content. The proteins (VEGF and TGF-β ) in the CM was found to be stable in the pH range of 5.0-8.0
In vitro analysis of conditioned media based formulation
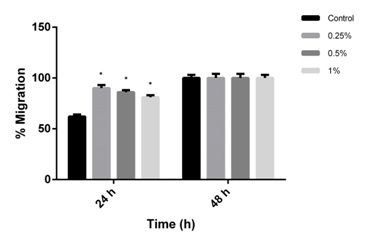 Figure 4: Effect of conditioned media based formulation (0.25%, 0.5% and 1%) on migration of human dermal fibroblasts as evaluated by scratch wound assay. The CM based formulation at all the concentration tested significantly promotes migration of cells towards wound area. The percentage of cell migration after 24 hrs was found to be 90%, 86% and 81% at concentration of 0.25%, 0.5%, 1% respectively when compared to control (62%) (mean±SEM, P<0.05). The experiments were repeated 2 times in duplicates.
Figure 4: Effect of conditioned media based formulation (0.25%, 0.5% and 1%) on migration of human dermal fibroblasts as evaluated by scratch wound assay. The CM based formulation at all the concentration tested significantly promotes migration of cells towards wound area. The percentage of cell migration after 24 hrs was found to be 90%, 86% and 81% at concentration of 0.25%, 0.5%, 1% respectively when compared to control (62%) (mean±SEM, P<0.05). The experiments were repeated 2 times in duplicates.The ability of the CM formulation to decrease UVB induced CPD formation was evaluated in photodamaged (UVB dose; 300 mJ/cm2) fibroblast cells after 48 h as compared to irradiated untreated control cells. Pyrimidine dimers are DNA lesions formed from thymine/cytosine bases in DNA viaphotochemical reactions on UVB exposure. The effect of the formulation at different concentrations (0.25%, 0.5% and 1%) was evaluated on the UVB irradiated photodamaged cells and the percentage decrease in CPD formation was compared with the UVB-irradiated untreated cells. The results showed that the CM based formulation led to a decrease in the CPD formation in the UVB irradiated cells compared to the untreated UVB irradiated cells. The formulation protected the cellular DNA from UV irradiation and reduced the CPD formation (Figure 5). The CM formulation showed higher protective effect at the concentration of 0.5% followed by 0.25% (42%±3.59 and 33%±3.68 decrease in CPD respectively) when compared to 1% (23%±4.21). But all the formulation concentrations showed a comparatively higher protective effect than the control UVB irradiated cells (18%±2.42). The unprotected UV rays are known to cause mutation in the DNA that may lead many pathological conditions such as skin cancers [22]. The CPD is a photoproduct of the damaged DNA and it is one of the most causative agents for primary lesions in UV-irradiated DNA [23]. The CPD assay confirms that the CM growth factors and the formulation were useful in preventing the CPD formation in the cells by UV irradiation.
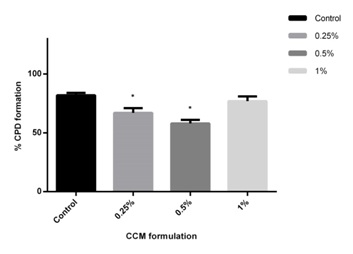 Figure 5: CPD formation assay in UV irradiated HFF-1 cells. The CM based formulation at concentration of 0.25% and 0.5% significantly reduced CPD formation wherein the percentage of CPD was lower in CM treated UVB irradiated HFF-1 cells (67% and 58%) as compared to UVB-irradiated untreated cells (82%) (mean±SEM, P<0.05). The experiments were repeated 2 times in duplicates.
Figure 5: CPD formation assay in UV irradiated HFF-1 cells. The CM based formulation at concentration of 0.25% and 0.5% significantly reduced CPD formation wherein the percentage of CPD was lower in CM treated UVB irradiated HFF-1 cells (67% and 58%) as compared to UVB-irradiated untreated cells (82%) (mean±SEM, P<0.05). The experiments were repeated 2 times in duplicates.In vitro evaluation of conditioned media based formulation
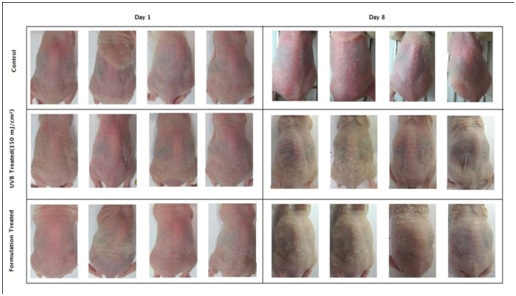 Figure 6: Macroscopic images of nude mice treated with UVB-irradiation and CM formulation after UVB irradiation: The skin surface was smooth and even in all the animals of respective groups on Day 1. On Day 8, the UVB- irradiated animals showed appearance of wrinkles and desquamation when compared to animals in the control group. The animals in the CM formulation treated group showed restoration of UVB induced damage (Day 8).
Figure 6: Macroscopic images of nude mice treated with UVB-irradiation and CM formulation after UVB irradiation: The skin surface was smooth and even in all the animals of respective groups on Day 1. On Day 8, the UVB- irradiated animals showed appearance of wrinkles and desquamation when compared to animals in the control group. The animals in the CM formulation treated group showed restoration of UVB induced damage (Day 8).The skin moisture level of the animals was measured with a corneometer on all the days (day 1 to 8) before the schedule of UVB exposure. The animals of non-irradiated control group displayed high mean corneometer readings ranging from 49.6±3.7 to 55.80±1.2 corneometer units. These values indicate good moisture capacities of the skin and reflect high hydration index. However, exposure of animals to UVB resulted in a decrease in corneometer readings beginning from very first exposure, which sequentially dropped further to a considerable extent till day 8th of study (54.60±4.7 to 14.5±1.3). This decrease in skin hydration manifested in the form of substantial extent of skin roughness as observed on day 8th of animals in UVB treated animals. The mean corneometer readings of the formulation-treated group ranged from 47.8±3.2 to 37.4±2.5corneometer units. A comparison of corneometer units on day 1 and day 8 revealed that there was a 72.32 % decrease in skin moisture content in UVB treated group. But, in the CM formulation-treatedgroup, the skin moisture reduction was only 21.75 % (Figure 7). The study showed evidence that the UVB exposure led to decreased moisture capacity in the skin of the animals. However, the formulation gave protection (69.92%) against the UVB rays and significantly decreased the skin moisture reduction from the UVB exposure.
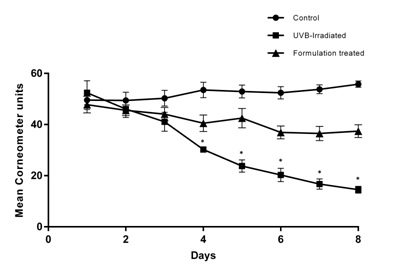 Figure 7: Mean corneometer reading and skin moisture reduction in nude mice model: The animals of non-irradiated control group displayed high mean corneometer readings (49.6 to 55.8) which reflect high hydration index and moisture capacity. The animals of UVB irradiated group showed decrease in the corneometer readings which dropped further sequentially (54.60 to 14.5). However, animals in the CM formulation treated group showed decreased skin moisture reduction significantly when compared to animals of UVB exposed group (47.8 to 37.4) (mean±SEM, P<0.05) (n=5).
Figure 7: Mean corneometer reading and skin moisture reduction in nude mice model: The animals of non-irradiated control group displayed high mean corneometer readings (49.6 to 55.8) which reflect high hydration index and moisture capacity. The animals of UVB irradiated group showed decrease in the corneometer readings which dropped further sequentially (54.60 to 14.5). However, animals in the CM formulation treated group showed decreased skin moisture reduction significantly when compared to animals of UVB exposed group (47.8 to 37.4) (mean±SEM, P<0.05) (n=5).The Erythema Index (E.I.) in the skin was evaluated using Mexameter every day (for 8 days) before UVB exposure. Mean E.I. was obtained on the dorsal skin of all the animals. An increase in mean E.I. from 286.8±12.9 to 366.2±14.4 was observed from day 1 to day 8 in UVB exposed group; whereas, the mean E.I. was lower in the formulation-treated group (265±14.3 to 271.4±15.9). A comparison of mean E.I values between days 1 and 8 reveals that UVB exposure led to more than 27.68% increase in E.I. whereas for the CM formulation treated group, the E.I was less than 3%. There was more than 25.68 % reduction of erythema in the CM formulation-treated group than the UVB-irradiated group (Figure 8). This experiment using Mexameter reveals that the CM formulation significantly prevented the increase in E.I. and protected (91.29%) the animal skin from the harmful UVB rays.
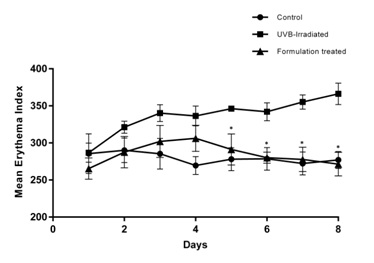 Figure 8: Mean Erythema Index (E.I.) in nude mice model: An increase in the mean Erythema Index (E.I.) was observed from Day 1 to Day 8 in animals of UVB irradiated group (286.8 to 366.2) whereas it was significantly lower in CM formulation treated group (265 to 271.4) (mean±SEM, P<0.05) (n=5).
Figure 8: Mean Erythema Index (E.I.) in nude mice model: An increase in the mean Erythema Index (E.I.) was observed from Day 1 to Day 8 in animals of UVB irradiated group (286.8 to 366.2) whereas it was significantly lower in CM formulation treated group (265 to 271.4) (mean±SEM, P<0.05) (n=5).The histological features and alterations in the skin of all the animals were determined by staining with H&E. Histological examination of each slide was done for epidermal thickness; hyperkeratosis and infiltration of inflammatory cells in the dermis. It was found that UVB exposure led to induction of pathological features in the skin associated with photo aging. Animals in UVB-irradiated group displayed a substantial increase in epidermal thickness (14.25±1.19 pixels as compared to 3.06±0.41 pixels in untreated control); increase in dermal thickness (99.21±8.73 pixels as compared to 55.18±3.64 pixels in untreated control); hyperkeratosis and infiltration of inflammatory cells in the dermis as compared to untreated control. A lower extent of epidermal hyperplasia as indicated by a reduced value of epidermal thickness (10.98±2.05 pixels as compared to 14.25±1.19 pixels in the UVB-irradiated group) and dermal thickness (76.38±4.60 pixels as compared to 99.21±8.73 pixels in the UVB-irradiated group) were recorded in CM formulation treated animals. Hyperkeratosis was low in CM formulation treated animal. The extent of infiltration of inflammatory cells in the dermis was found to be moderate in all CM formulation treated animals (Figure 9a and Table 1). In addition, collagen degradation in UVB induced mice as compared to CM formulation treated UVB induced mice was evaluated by Masson trichrome staining. Analysis of intensity of blue color, indicative of collagen content revealed that UVB-irradiation caused collagen fiber destruction, but CM formulation treatment ameliorated this effect (Figure 9b). Taken together, the detailed histological evidence of the skin proved that the formulation with CM as its active ingredient was able to protect the skin from the UVB induced damage in the in vivo system.
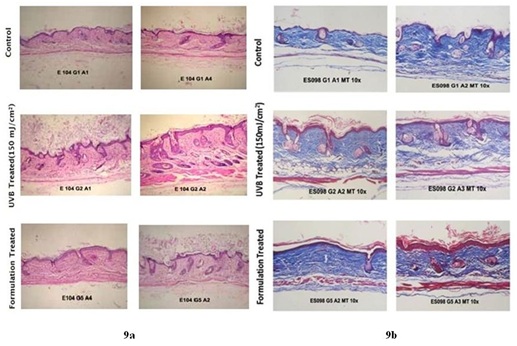
9a) Photomicrographs of skin tissue sections stained with standard Hematoxylin and Eosin stain. Histological analysis showed that the animals in the UVB irradiated group displayed substantial increase in the epidermal (14.25 pixels as compared to 3.06 pixels in control group) and dermal thickness (99.21 pixels as compared to 55.18 pixels in control group), hyperkeratosis and infiltration of inflammatory cells in the dermis as compared to non-irradiated control group. The animals treated with CM formulation exhibited lower extent of the epidermal thickness and significant decrease in dermal thickness (10.98 pixels and 76.38 pixels as compared to 14.25 pixels and 99.21 pixels respectively in UVB treated group respectively)(mean±SEM, P<0.05) (n=5).
9b) Photomicrographs of skin tissue sections stained with Masson Trichrome stain. Collagen staining appears blue. There was less extent of collagen degradation in CM formulation treated UVB induced mice when compared to UVB irradiated group.
|
Group |
Mean Epidermal Thickness (Pixels* ± SEM) |
Mean |
Epidermal hyperplasia |
Hyperkeratosis |
Infiltration of inflammatory cells in dermis |
|
Control |
3.06 ± 0.41 |
55.18± 3.64 |
A |
A |
A |
|
UVB-irradiated |
14.25 ± 1.19 |
99.21± 8.73 |
+++ |
+++ |
+++ |
|
Formulation treated |
10.98 ± 2.05 |
76.38± 4.60 |
+ |
+ |
++ |
The UVB exposure to the normal skin leads to photoaging and causes various changes in the skin architecture. Photoaging depends primarily on the degree of sun exposure and skin pigment. The photo-damaged skin is associated with increased epidermal thickness and alterations in dermis such as erythema, scabs, roughness, and wrinkling of the skin. Wrinkle formation occurs because of accumulated skin damages such as matrix destruction and skin inflammation.
In the present study, the experiments showed evidence that the UVB irradiation for 7 consecutive days led to change in the macroscopic and microscopic levels. Macroscopically UVB exposure led to an increase in the number and depth of wrinkles, loss of moisture content, induction of erythema. Whereas microscopically UVB led to epidermal hyperplasia, hyperkeratosis and an increase in infiltration of inflammatory cells in the dermis as compared to the untreated group animals. These UVB induced toxic effects were reversed by the stem cell CM based formulation. It was found that topical application of CM formulation led to the restoration of UVB induced effects at macroscopic and microscopic levels. Macroscopically the CM formulation led to smoother skin, a decrease in erythema as well as considerable restoration of moisture content in animals. While microscopically photoprotective effects of the CM formulationwere supported by the low extent of hyperkeratosis, epidermal hyperplasia, infiltration of inflammatory cells and lesser collagen degradation as compared to animals exposed to UVB irradiation.
CONCLUSION
ACKNOWLEDGEMENT
CONFLICT OF INTEREST
REFERENCES
- Bowen RL, Atwood CS (2004) Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology 50: 265-290.
- Bouwstra JA, Ponec M (2006) The skin barrier in healthy and diseased state. Biochim Biophys Acta 1758: 2080-2095.
- Luebberding S, Krueger N, Kerscher M (2013) Age-related changes in skin barrier function - quantitative evaluation of 150 female subjects. Int J Cosmet Sci 35: 183-190.
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC (2012) Skin anti-aging strategies. Dermatoendocrinol 4: 308-319.
- Trojahn C, Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J (2015) Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int 2015: 318586.
- de Gruijl FR (2000) Photocarcinogenesis: UVA vs UVB. Methods Enzymol 319: 359-366.
- D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T (2013) UV radiation and the skin. Int J Mol Sci 14: 12222-12248.
- Flament F, Bazin R, Laquieze S, Rubert V, Simonpietri E, et al. (2013) Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol 6: 221-232.
- Pawitan JA (2014) Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014: 965849.
- Balasubramanian S, Thej C, Walvekar A, P. Swamynathan P, Gupta PK, et al. (2017) Evaluation of the secretome profile and functional characteristics of human bone marrow mesenchymal stromal cells-derived conditioned medium suggest potential for skin rejuvenation. Journal of Cosmetics, Dermatological Sciences and Applications 7: 99-117.
- Kwon TR, Oh CT, Choi EJ, Kim SR, Jang YJ, et al. (2015) Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol Photoimmunol Photomed 32: 120-128.
- Liu Q, Luo Z, He S, Peng X, Xiong S, et al. (2013) Conditioned serum-free medium from umbilical cord mesenchymal stem cells has anti-photoaging properties. Biotechnol Lett 35: 1707-1714.
- Sohn SJ, Yu JM, Lee EY, Nam YJ, Kim J, et al. (2018) Anti-aging Properties of Conditioned Media of Epidermal Progenitor Cells Derived from Mesenchymal Stem Cells. Dermatol Ther (Heidelb) 8: 229-244.
- Li M, Zhao Y, Hao H, Dong L, Liu J, et al. (2017) Umbilical cord-derived mesenchymal stromal cell-conditioned medium exerts in vitro antiaging effects in human fibroblasts. Cytotherapy 19: 371-383.
- Muthukumar A, Seetharam RN (2016) Stem Cell Derived Cosmetic Products: An overview. MJMS 1: 46-52.
- Jayaraman P, Nathan P, Vasanthan P, Musa S, Govindasamy V (2013) Stem cells conditioned medium: a new approach to skin wound healing management. Cell Biol Int 37: 1122-1128.
- Park CW, Kim K-S, Bae S, Son HK, Myung P-K, et al. (2009) Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2: 59-68.
- Rolandsson Enes S, Ahrman E, Palani A, Hallgren O, Bjermer L, et al. (2017) Quantitative proteomic characterization of lung-MSC and bone marrow-MSC using DIA-mass spectrometry. Sci Rep 7: 9316.
- Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, et al. (2007) Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics 6: 1680-1689.
- Amirthalingam M, Kasinathan N, Amuthan A, Mutalik S, Sreenivasa Reddy M, et al. (2016) Bioactive PLGA-curcumin microparticle-embedded chitosan scaffold: in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol 45: 233-241.
- Amirthalingam M, Kasinathan N, Mutalik S, Udupa N (2015) In vitro biocompatibility and release of curcumin from curcumin microcomplex-loaded chitosan scaffold. J Microencapsul 32: 364-371.
- Matsumura Y, Ananthaswamy HN (2004) Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 195: 298-308.
- You YH, Lee DH, Yoon JH, Nakajima S, Yasui A, et al. (2001) Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J Biol Chem 276: 44688-44694.
Citation: Amirthalingam M, Bhat S, Dighe PA, Seetharam RN (2019) Human Mesenchymal Stromal Cells-Derived Conditioned Medium Based Formulation for Advanced Skin Care: in vitro and in vivo Evaluation. J Stem Cell Res Dev Ther 5: 012.
Copyright: © 2019 Muthukumar Amirthalingam, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

