
A Comparison of the Toxicity of Calcium and Sodium Hypochlorite against Culex Pipiens (Diptera: Culicidae) Larvae
*Corresponding Author(s):
Shahen MZoology Department, Faculty Of Science, Tanta University, Tanta, Egypt
Tel:+20 1014260584,
Email:Mshahen@science.tanta.edu.eg
Abstract
The larvicidal activities of sodium and calcium hypochlorite against the filaria vector Culex Pipiens were investigated under laboratory conditions. The different larval instars were exposed to different concentrations of sodium and calcium hypochlorite and the lethal concentrations were estimated. Calcium hypochlorite was more toxic to all larval instars than sodium hypochlorite. The estimated LC50 values of sodium hypochlorite for 1st, 2nd, 3rd and 4th instar larvae, were 12.24, 46.2, 65.33 and 99.5 ppm, respectively. Meanwhile, the LC50 values of calcium hypochlorite were 1.3, 5.75, 8.7 and 10.6 ppm for 1st, 2nd, 3rd and 4th larval instars, respectively. Both compounds caused mortality in all larval instars. Calcium hypochlorite was more effective than sodium hypochlorite when mosquito larvae were exposed from the start of the 1st instar onwards. Hypochlorite compounds significantly reduced larval survival rates and induced significant prolongation in the duration of larval instars and development period. Larvae treated with sublethal concentrations of hypochlorite compounds suffered from severe inhibition of integument development which was very fragile and ruptured easily. Most of treated larvae failed in splitting their exuviae and were unable to liberate themselves from the larval cuticle. Thesiphon of most treated larvae was wider and paddle filled with black patches.
Keywords
Biological control; Culex Pipiens; Filariasis; Hypochlorite; Toxicity
INTRODUCTION
Culex pipiens is the most common and widely distributed mosquito in Egypt [1-3]. It has been recognized as primary vector for Wuchereriabancrofti, one of the major public health problems in Egypt [4-6]. It is also the vector of Rift valley fever virus [7], West Nile and Sindbis viruses [8,9], as well as serious nuisance pests. It is the most common mosquito species and breeds primarily in large numbers in receptacles that hold stagnant water and organic materials such as drainage cesspools, irrigation canals, sewage streams and pit latrines [3,10,11].
In an effort to reduce the spread of these vectors, control measures must be taken. Mosquito control represents an important strategy for prevention of disease transmission and epidemic outbreaks. Three major control measures are used to eliminate mosquitoes; adulticiding, source reduction and larviciding [12] eggs [13]. Where source reduction is not possible, there are several types of larvicides. Including concentrations of certain types of bacteria Bacillus thuringiensis, monomolecular surface films, oils, and chemical growth inhibitors [14,15]. Adulticiding is the least efficient method of mosquito control because it is nonspecific and may have negative impact on other organisms [16,17]. Recently, intensive and continued use of insecticides to control mosquitoes has caused the development of resistance of mosquitoes to every conventionally used chemical class including organochlorine and organophosphate insecticides, microbial drugs and insect growth regulators and hindered the control programs [18,19]. The abundant use of insecticides might also impact fish, amphibians, aquatic arthropods, and other aquatic organisms. The development of environmentally friendly insecticides having specificity to insects along with low toxicity to vertebrates has attracted worldwide attention of scientists [20,21].
Sodium hypochlorite, the active ingredient in house hold bleach, has been used to disinfect drinking and waste water prior to discharge [22] and sanitizer because of its high effectiveness and wide spectrum action against bacteria, viruses, fungi, and algae [23]. Chlorinated lime has been largely used for the control of cercariae in irrigation canal and ditches in Egypt [3,22]. Previous researchers have shown that sodium hypochlorite was lethal for immature stages of mosquitoes. [12] found that 100ppm of chlorine dosage killed Aedes aegypti in 24 hours in both pure and water from a larval habitat. [24] In series of studies on community participation in dengue prevention in Hondurans used household detergent to kill Aedes aegypti eggs. They investigated the lethal dose required to achieve 50% mortality in larvae, pupae, and eggs. Mortality (50%) of larvae was achieved between 27min and 4h, and between 4.3h and 15h for the pupae using a concentration between 100-2.600ppm in clean water. Eggs treated with 100 ppm did not hatch [25] reported the use of 1% and 5% NaOCl to kill 100% of Aedes aegypti larvae in less than 24 h as part of program to eliminate Aedes aegypti introduced into Australia. [26] proved that 25% dilution of the sodium hypochlorite mother solution (15g/100ml) was capable of destroying the chorion of the eggs of Aedesalbopicus in less than 1 min. Sodium hypochlorite is already used to remove the chorion of any insect egg in embryological and genetic research [3,27]. The concentration of bleach that was required to kill all immature was higher in the presence of food and older immature. Nothing is known about the effect of hypochlorite on Culex pipiens.
The acute toxicity of insecticides is used to produce mortality in target insect population withina given area. Treatment of target species with an insecticide may be uneven due to many factors, with some individuals therefore receiving sublethal dosages of insecticides. Sublethal dosages of insecticides may affect insect population by: 1) affecting survival, 2) affecting the reproductive ability of individuals or 3) affecting the genetic makeup of future generations [28-30]. Insecticides have been shown to affect a number of reproductive parameters in mosquitoes, including fecundity [31,32], egg hatching [33,34], immature development [35], adult longevity adult size [36] and blood feeding [37,38].
There are no reports on the effect of hypochlorite compounds on the toxicity in Culex pipiens. Therefore, the present study was planned with the objective to understand the toxicity of hypochlorite of these compounds on Culex pipiens, when exposed to sublethal concentration levels, which usually occur after field application of the compounds.
The aim of the study was to evaluate the influence of two hypochlorite compounds (sodium and calcium hypochlorite) on the Culex pipiens immature stages under laboratory condition through:
Determination of the lethal effects of hypochlorite compounds on Culex pipiens immature stages.
Determination of the survival rates of different immature stages of Culex pipiens exposed to sublethal concentrations of sodium and calcium hypochlorite.
MATERIALS AND METHODS
Culex pipiens source and maintenance
Culex pipiens was initially obtained from larval breeding site at Tanta City, Egypt. Larvae collected were transferred to a plastic whirl-pack bags (Nasco) half-filled with water from the breeding place and brought to the laboratory. In the laboratory, fourth in star larvae were collected and identified using the proposed key of [3, 39] for Egyptian culicine mosquitoes.
Larvae were reared in plastic pans containing dechlorinated tap water. They were fed dry fish food flakes (Tetra Min) sprinkled on the water. Pupae were collected, transferred to a small plastic cups half field with water, and introduced into 30 cm cubic wooden emergence cages covered with mesh gauze.
Emerged adults had continuous access to 10% sucrose solution. Two days after adult emergence, females were blood fed with pigeon and were then provided with containers half filled with water for oviposition. Egg rafts were collected and placed in small plastic cups (10cm in diameter and 7cm deep) filled with water. Larvae hatched from these rafts were transferred to wide plastic pans filled with water and were reared as previously described.
The mosquito colony was maintained in the insectary room at the Zoology Department, Faculty of Science, Tanta University at 27-30°C and approximately 70% humidity. All experiments were conduction on the F1 generation of the field collected 4th instar larvae.
Chemicals
Two chemical compounds containing high reactive nascent oxygen were tested against Culex pipiens: Calcium hypochlorite (also known as chlorinated lime and bleaching powder) manufactured by Egypt Corporation for Chemical Industries, El-Max, Alexandria, Egypt (active ingredient of approximately 38%), and the commercial bleaching solution, sodium hypochlorite (active ingredient of approximately 5-6%) manufactured by Alexandria Corporation for Oils and Soap, Kafr El-Zayait, Egypt [3].
Bioassay
The bioassay of the LC50 was performed according to the standard methodology of [40]. A series of dilutions using dechlorinated tap water ranged between 4 and 500ppm for sodium hypochlorite and 1 and100ppm for calcium hypochlorite were prepared using the stock solution. In the bioassay test, 50 larvae of 1st, 2nd, 3rd and 4th were used separately per each concentration and control with 3 replicates. The larvae were placed in a plastic pan containing 1000ml of each test solution. Dechlorinated tap water was used in the control groups. Dry fish food flakes (Tetra Min) were added to each container. The experiment was conducted at 27-30°C and 70% relative humidity. The mortality rates were recorded after 24h.
Data analysis: Probit analysis [41] was used to analyze data and to determine the 50% lethal concentration (LC50) and the 90% lethal concentration (LC90) values using dosage-mortality regression line.
Effect of sub lethal concentrations of sodium and calcium hypochlorite on Culex pipiens egg hatching and larval development
In this experiment, the comparative effect of sodium and calcium hypochlorite at their sublethal concentrations on egg hatching, the growth and survival rates of Culex pipiens larvae was investigated. Groups of 100 eggs and of each 1st, 2nd, 3rd and 4th larval instars were placed separately in a pan containing 2000ml of sublethal concentrations determined for each larval instar in the previous bioassay test. The sublethal concentrations ranged between 4 and 100 ppm for sodium hypochlorite and between 0.1 and 20 ppm for calcium hypochlorite. Control experiments were conducted using dechlorinated tap water. There were three replicates for each concentration and control. The larvae were provided with a diet of dry fish food flakes (Tetra Min). After 24 h of exposure, the survivors were counted rinsed with tap water and transferred to rearing pans containing dechlorinated tap water. There was no fixed time of observation; observation was sustained from the onset of the experiment until larval death or else of successful adult emergence. The larvae were fed dry fish food flakes (Tetra Min) for the duration of the experiment. The experiment was conducted at 27-30°C and 70% relative humidity.
Data on growth and survival percentage were collected and the survival curves were used for comparison using the program of Graphpad prism. Data for growth and survival rates were subjected to One-way analysis of variance (ANOVA) with Dunnett's posttest using Graph Pad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA to determine the effects of sublethal concentrations of hypochlorite compounds on growth and survival of mosquito immature stages.
Morphological studies
The objective of this study was to prepare whole mount slides of larvae exposed to 1/10 LC50 and LC50 of sodium and calcium hypochlorite for 24h according to the method described by [42]. Larvae were placed on a glass slide with the dorsal side up. The siphon lies to the right side of the slide. Excess water was removed with a filter paper. Birllis, gum chloral hydrate medium was placed on the specimen. The preparation was covered with a glass cover slip and allowed to dry.
RESULTS
Susceptibility of Culex pipiens immature to calcium and sodium hypochlorite
The purpose of this study was to investigate the susceptibility of the four larval instars of Culex pipiens to calcium and sodium hypochlorite. The susceptibility of the different larval instars to these compounds is presented in (Table 1). In general, all larval instars were more susceptible to calcium hypochlorite than sodium hypochlorite. In particulars the 1st instar larvae were more susceptible to calcium hypochlorite than sodium hypochlorite. The estimated LC50 values for 1st instar larvae exposed to calcium and sodium hypochlorites were 1.3±0.04 and 12.24±0.43ppm, respectively. The LC90 values indicated that calcium hypochlorite was more toxic to 1st instar than sodium hypochlorite with LC90 values of 2.65±0.19 and 24.63±3.43ppm, respectively (Figure 1).
|
Larval Instars |
LC50 ± SD |
LC90 ± SD |
||
|
Sodium Hypochlorite |
Calcium Hypochlorite |
Sodium Hypochlorite |
Calcium Hypochlorite |
|
|
1st |
12.24 ± 0.43 (10.3, 14.23) |
1.3 ± 0.04 (0.37, 2.23) |
24.63 ± 3.43 (22.8, 26.5) |
2.65 ± 0.19 (2.1, 3.21) |
|
2nd |
46.2 ± 1.62 (44.7, 47.7) |
5.75 ± 0.19 (4.58, 6.92) |
97.86 ± 10.98 (96, 99.8) |
11.90 ± 1.96 (10.7, 13.14) |
|
3rd |
65.33 ± 1.25 (64.1, 66.6) |
8.7 ± 0.55 (7.45, 10) |
195.24 ± 3.29 (93.2, 97.3) |
35.6 ± 9.3 (33.3, 37.9) |
|
4th |
99.5 ± 5.24 (97.4, 101.6) |
10.6 ± 0.45 (9.1, 12.1) |
301 ± 5.93 (298, 304) |
60.70 ± 0.02 (58.8, 62.6) |
Table 1: Mortality response of different larval instars of Culex pipiens to sodium and calcium hypochlorite 24h post exposure.
* LC50 and LC90 values are expressed as ppm.
** Values between brackets are confidence limits.
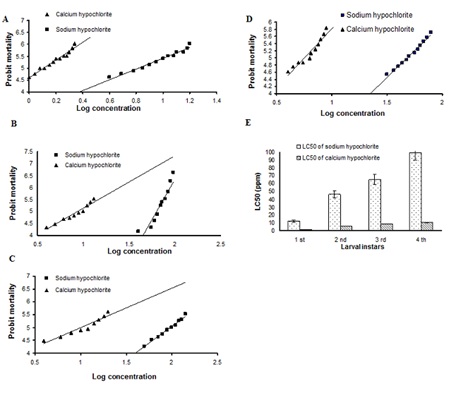 Figure 1: The 24-h concentrations - mortality regression lines for sodium and calcium hypochlorite against larvae of Culex pipiens. (A) First instar (B) Second instar (C) Third instar (D) Fourth instar (E) The 24-h Concentrations - mortality diagram for sodium and calcium hypochlorite against the four larval instars of Culex pipiens.
Figure 1: The 24-h concentrations - mortality regression lines for sodium and calcium hypochlorite against larvae of Culex pipiens. (A) First instar (B) Second instar (C) Third instar (D) Fourth instar (E) The 24-h Concentrations - mortality diagram for sodium and calcium hypochlorite against the four larval instars of Culex pipiens.
A similar trend was observed for 2nd instar larvae, they were more susceptible to calcium hypochlorite than sodium hypochlorite. The estimated LC50 values for 2nd instar larvae exposed to calcium and sodium hypochlorite were 5.75±0.19 and 46.2±1.62 ppm, respectively. The LC90 values obtained indicated again that calcium hypochlorite was more toxic to 2nd instar larvae than sodium hypochlorite with LC90 values of 11.90±1.96 and 97.86±10.98ppm respectively (Figure 1).
The LC50 values of calcium and sodium hypochlorites after exposure of 3rd instar larvae were 8.7±0.55 and 65.3±3 1.25ppm respectively. Calcium hypochlorite was more toxic to 3rd instar larvae than sodium hypochlorite with LC90 values of 35.6±9.3 and 195.24±3.29 ppm respectively (Figure 1).
For 4th instar larvae, the estimated LC50 values for calcium and sodium hypochlorite treatments were 10.6±0.45 and 99.5±5.24 ppm, respectively. This result indicated clearly that 4th instar larvae were more susceptible to calcium hypochlorite than sodium hypochlorite. The LC90 value obtained for calcium hypochlorite indicated that this compound was more toxic to 4th instar larvae than sodium hypochlorite with LC90 values of 60.70±0.02 and 301±59.3 ppm respectively (Figure 1).
In general, first instar larvae were more susceptible to calcium hypochlorite than 2nd, 3rd, and 4th instar larvae with 4.4, 6.6 and 8.2-fold differences, respectively (Figure 1).
Effect of sublethal concentrations of sodium and calcium hypochlorite on Culexpipiens egg hatching and larval development
The present work investigated the effect of sublethal concentrations of sodium and calcium hypochlorite, estimated in the above experiments, on Culex Pipiens egg hatchingandlarval survival rates and possible alterations of their morphology and histology.
Effect of sodium hypochlorite on Culex pipiens egg hatching and development: The hatching rate of the control eggs was 32%. Control first instar larvae were able to reach the adult stage in 20 days with a survival percentage of 32% (Figure 2). When eggs were inoculated into water treated with 4 and 12ppm sodium hypochlorite concentrations, their survival rates were 5% and 3%, respectively. At 20ppm concentration, the larvae that reached the 3rd instar were unable to complete their development. Larvicidal effects were observed when the eggs were inoculated into water treated with 28 and 36ppm sodium hypochlorite concentrations. All hatched larvae died during the 1st instar.
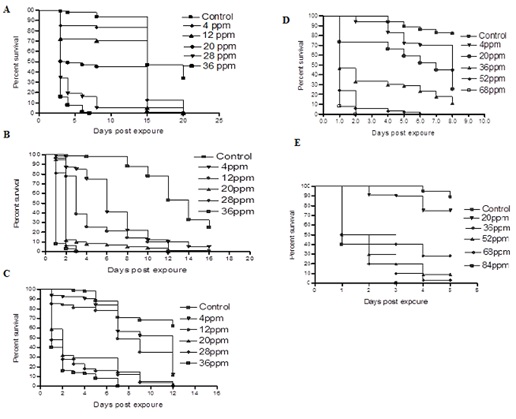 Figure 2: Survival of Culex pipiens. (A) Eggs (B) 1st Larval instar (C) 2nd larval instar (D) 3rd larval instar (E) 4th larval, exposed to sublethal concentrations of sodium hypochlorite. Controls were reared in dechlorinated tap water. Hundred eggs were used /concentration. Sublethal concentrations selected were 4-36ppm. Survival of larvae was estimated by quantifying the proportion of larvae reaching the adult stage.
Figure 2: Survival of Culex pipiens. (A) Eggs (B) 1st Larval instar (C) 2nd larval instar (D) 3rd larval instar (E) 4th larval, exposed to sublethal concentrations of sodium hypochlorite. Controls were reared in dechlorinated tap water. Hundred eggs were used /concentration. Sublethal concentrations selected were 4-36ppm. Survival of larvae was estimated by quantifying the proportion of larvae reaching the adult stage.
Effects of sodium hypochlorite on the survival rate and development of Culex pipiens larval instars
Effects on 1st larval instar: The mean percentage survivals and development of 1st larval instar of Culex pipiens after exposure to different sublethal concentration of sodium hypochlorite (0-36ppm) are illustrated in (Figure 2). Control 1st instar larvae required 18 days to reach the adult stage with survival percentage of 25%. At 4 and 12ppm, the survival percentages of larvae significantly deceased (5% and 2%, respectively) as compared to the control group. At 20ppm, all treated larvae that reached the 3rd instar eventually died. Meanwhile at 28 and 36ppm all larvae succumbed during the 2nd instar.
Effects on the 2nd larval instar: Control 2nd instar larvae required 14 days to reach adulthood with survival percentage of 62% (Figure2C). At 4 and 12ppm concentrations, the survival percentages significantly decreased and were similar (11%). At 20ppm concentration, the survival percentage of larvae significantly decreased (2%) as compared to the control insects. At 28ppm concentration, all treated larvae failed to reach the pupa stage, while at 36 ppm concentration most larvae died during the 3rd instar.
Effects on the 3rd larval instar: Control 3rd instar larvae reached the adult stage in 8 days with survival percentage of 72% (Figure 2). At 4, 20 and 36ppm concentrations, the survival percentages were 43, 6 and 4%, respectively. At 52ppm concentration, all exposed larvae failed to reach the pupa stage, while at 68ppm concentration most larvae died without molting to 4th instar.
Effects of on the 4th larval instar: Control 4th instar larvae required 5 days to reach the adult stage with survival percentage of 89% (Figure 2). At 20, 36, 52 and 68ppm concentrations, the survival rates were 75, 28, 9 and 3 % respectively Fourth instar larvae exposed to 84ppm concentration failed to pupate.
Effects of calcium hypochlorite on egg hatching and development of Culex Pipiens: In control experiment, 42% of Culex pipiens eggs hatched and the hatched larvae were able to reach the adult stage in 20 days with survival percentage of 42% (Figure 3). At 1, 2 and 3ppm concentrations, most of treated eggs hatched to larvae, however further development was inhibited. Larvicidal effects were detected when eggs were inoculated into water treated with 4 and 5 ppm; most of the eggs failed to hatch and hatched larvae died during the 1st instar.
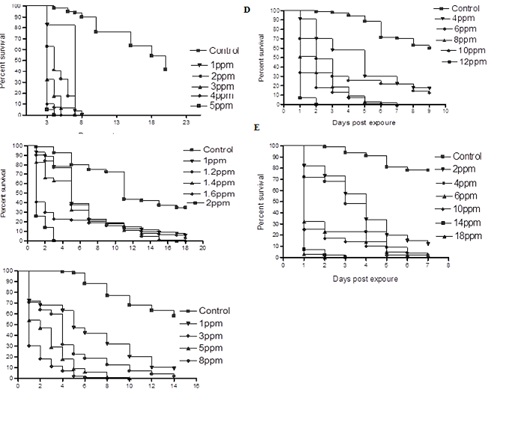 Figure 3: Survival of Culex pipiens. (A) Eggs (B) 1st larval instar (C) 2nd larval instar (D) 3rd larval instar (E) 4th larval, exposed to sublethal concentrations of calcium hypochlorite. Controls were reared in dechlorinated tap water. Hundred eggs were used /concentration. Sublethal concentrations selected were1-5ppm. Survival of larvae was estimated by quantifying the proportion of larvae reaching the adult stage.
Figure 3: Survival of Culex pipiens. (A) Eggs (B) 1st larval instar (C) 2nd larval instar (D) 3rd larval instar (E) 4th larval, exposed to sublethal concentrations of calcium hypochlorite. Controls were reared in dechlorinated tap water. Hundred eggs were used /concentration. Sublethal concentrations selected were1-5ppm. Survival of larvae was estimated by quantifying the proportion of larvae reaching the adult stage.
Effects on 1st larval instar: Control 1st instar mosquitoes required 18 days to reach adulthood with survival percentage of 48 % (Figure 3). The survival rates of larvae treated with 1 and 1.2ppm significantly decreased (6 and 3%, respectively) as compared with the control insects. Larvae exposed to 1.4 and 1.6ppm developed to the 3rd instar and eventually died. At 2ppm concentration, most of the larvae succumbed during the 1st instar.
Effects on 2nd larval instar: Control 2nd instar larvae developed to the adult stage in 14 days with survival percentage of 43% (Figure 3). At 1ppm concentration, the survival rate was 9%, whereas at 3ppm concentration, only 2% of the larvae were able to reach the adult stage. Inhibition of development was observed when larvae were exposed to 5ppm and 8ppm concentrations; none of the treated larvae molted to 3rd instar.
Effects on the 3rd larval instar: Control 3rd larvae required 9 days to reach adulthood with survival percentage of 64% (Figure 3). Survival rates of larvae exposed to 4 and 6 ppm significantly decreased (17 and 11%, respectively) as compared to the control group. Larvae exposed to 8 and 10ppm died during the 4th larval instar whereas all larvae exposed to 12ppm succumbed during the 3rd instar.
Effects on the 4th larval instar: Under experimental conditions, control 4th instar larvae required 7 days to develop to the adult stage with survival percentage of 7% (Figure 3). Fourth instar larvae exposed to 2ppm concentration showed significantly lower survival percentage of 12% as compared to the control group, while exposure of larvae to 4ppm concentration gave very low survival percentage of only 2% as compared to the control insects. Similar figure was also recorded with 6 ppm larval treatment. All larvae exposed to 10 and 14 ppm concentrations died during the pupae stage whereas all larvae exposed to 18ppm concentration succumbed during the fourth instar.
Morphological changes induced by exposure of Culex pipiens larvae to sublethal concentrations of sodium and calcium hypochlorite
Larvae treated with sublethal concentrations of sodium and calcium hypochlorite differed from control larvae not only in their size, but also with regard to their integument, which was very fragile and ruptured easily than observed for any of the controls or any other larvae of the same culture stock (Figure 4). Furthermore, the siphon of most treated larvae was wider as compared to controls (Figure 5). All larval instars that were exposed to sublethal concentration of sodium and calcium hypochlorite showed black patches in the paddle (Figure 6). These patches appeared thicker and intense in larvae exposed to LC50 of sodium and calcium hypochlorite (Figure 6).
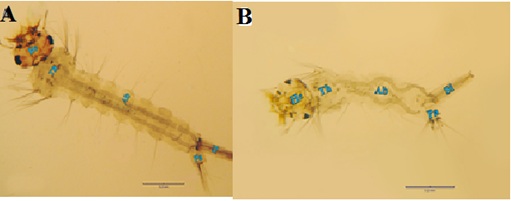 Figure 4: Morphogenetic changes found in Culex pipiens treated with LC50 of calcium hypochlorite at the 4th larval instar comparing to the control. (A) Normal 4th instar larva (He, head, Th, thorax, Ab, abdomen, Si, siphon, Pa, paddle). (B) Calcium and sodium hypochlorite-treated 4th instar larva, note the deformed soft larval integument and distortion of the abdominal segments. Such larva was unable to liberate themselves from the larval exuvium, and succumbed.
Figure 4: Morphogenetic changes found in Culex pipiens treated with LC50 of calcium hypochlorite at the 4th larval instar comparing to the control. (A) Normal 4th instar larva (He, head, Th, thorax, Ab, abdomen, Si, siphon, Pa, paddle). (B) Calcium and sodium hypochlorite-treated 4th instar larva, note the deformed soft larval integument and distortion of the abdominal segments. Such larva was unable to liberate themselves from the larval exuvium, and succumbed.
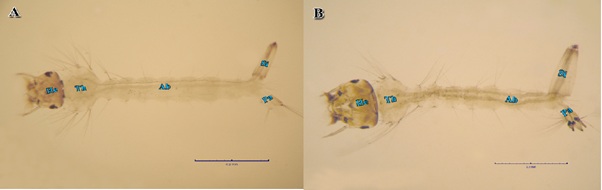 Figure 5: Morphogenetic changes found in Culex pipiens treated with LC10 of sodium and calcium hypochlorite at the 2nd larval instar comparing to the control. (A) Normal 2nd instar larva. (B) Sodium hypochlorite-treated 2nd instar larva, note the siphon of most treated larvae was wider as compared to controls.
Figure 5: Morphogenetic changes found in Culex pipiens treated with LC10 of sodium and calcium hypochlorite at the 2nd larval instar comparing to the control. (A) Normal 2nd instar larva. (B) Sodium hypochlorite-treated 2nd instar larva, note the siphon of most treated larvae was wider as compared to controls.
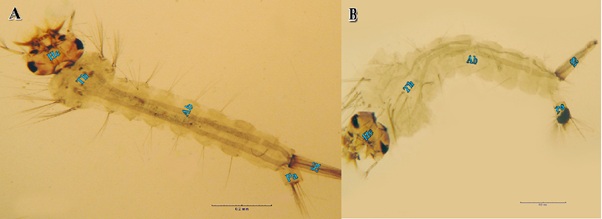 Figure 6: Morphogenetic changes found in Culex pipiens treated with LC50 of sodium hypochlorite at the 4th larval instar comparing to the control. (A) Normal 4th instar larva. (B). sodium and calcium hypochlorite-treated 4th instar larva, note black patches in the paddle.
Figure 6: Morphogenetic changes found in Culex pipiens treated with LC50 of sodium hypochlorite at the 4th larval instar comparing to the control. (A) Normal 4th instar larva. (B). sodium and calcium hypochlorite-treated 4th instar larva, note black patches in the paddle.
DISCUSSION
The purpose of this work was to determine the dose-response relationship and hence the concentrations of sodium and calcium hypochlorite compounds that are required to kill 50% (LC50) and 90% (LC90) of the different Culex pipiens larval instars.
Susceptibility of Culex pipiens larvae to hypochlorite compounds
The results obtained showed that hypochlorite compounds had different effects on the larval stages of Culex pipiens. Mortality rates of larvae varied according to the concentration and the type of hypochlorite compound. Calcium hypochlorite was more effective against all larval stages than sodium hypochlorite, where the median lethal concentrations (LC50) for sodium hypochlorite were 12.2±1.99ppm, 46.2±1.5 ppm, 65.33±1.2ppm and 99.5±2.06ppm, for 1st, 2nd, 3rd and 4th larval instars, respectively. While the LC50 values for calcium hypochlorite were 1.3±0.93ppm, 5.75±1.17ppm, 8.7±1.25ppm and 10.6±1.47ppm for 1st, 2nd, 3rd and 4th larval instars, respectively. Toxicity of hypochlorite compounds to Culex pipiens in this study was generally as those of [43] who found that the 100ppm sodium hypochlorite dosage killed Aedes aegypti larvae in 24h. Similarly, [24,44] found that 100-2600ppm of sodium hypochlorite in clean water was required to achieve 50% mortality in larvae, pupae and eggs of Aedes aegypti in a community participation research project in Honduras. [12] found that a concentration 250ppm NaOCl was able to kill all larvae of Aedes aegypti present at the time of application. [12] Found that the concentration of bleach that was required to kill all immature was higher in the presence of larval food and older immature.
In this study, the younger instars (1st and 2nd) were more susceptible to hypochlorite compounds than older ones (3rd and 4th instars). The susceptibility to exposure to hypochlorite declined with the age of mosquito larvae. It was suggested that the insecticides might vary in their rates of reaching the target site in different age groups [45]. Several reports demonstrated that age-dependent enzyme activity may be the reason for the present finding. Age -dependent enzyme activity had been reported on glutathione S-transferase in Aedes aegypti [46,47] and Malathion carboxylesterase in Aedesstephensimosquitoes [47,48].
Effect of sublethal concentrations of sodium and calcium hypochlorite on Culex pipiens egg hatching and larval development
The results indicated that hypochlorite compounds induced inhibition of growth of Culex pipiens larvae. There was a delay in the development of larvae to the pupal stage and some failed at the pupal stage when the 2nd and 3rd instars were exposed to hypochlorite compounds. This was specially noted when larvae were exposed to calcium hypochlorite. Data obtained revealed that when Culex pipiens eggs were inoculated into water treated with 4 and 12ppm sodium hypochlorite concentrations, the survival rates were very low (5% and 3%, respectively). The decreased rate of survival of larvae hatched from treated eggs may indicate that the lipid layer of the egg chorion, which provides a general barrier to hydrophilic materials [49-51], was damaged to the extent that the hypochlorite compounds targeted the embryo within the egg. Sodium hypochlorite is already widely used to remove the chorion of many insect eggs in embryological and genetic research [52,53]. The chorion is susceptible to desiccation and un-resistant to detergents or reducing agents as SDS [54,55].
Morphological changes induced by exposure of Culex pipiens larvae to sublethal concentrations of sodium and calcium hypochlorite
In the present study, differed larval instars of Culex pipiens treated with sublethal concentrations of sodium and calcium hypochlorite showed fragile integument than observed for any of the control larvae. The siphon of most treated larvae was wider and paddles with black patches these patches appeared thicker and intense in larvae exposed to LC50 of sodium hypochlorite. Abnormalities observed in larvae appear to be connected to increased internal body pressure and deterioration of the mechanical properties of the cuticle. Plantextracts tested against the 2nd instar larvae of Culex Pipiens induced some morphological abnormalities in pupae and adults (pupal-adult intermediates) [56,57].
The treatment of larvae of Culex pipiens with sublethal concentrations of sodium and calcium hypochlorite caused degeneration of the hypodermal cell layer structure of the alimentary canal, the mid gut cells appeared thinner and flattened, with disappearance of the peritrophic membrane, which might reduce food digestion and absorption, with concurrent reduction of the growth and the survival of larvae. Treated larvae showed reduced fat body, muscle, and Malpighian changes. A survey of the literature failed to reveal studies in which the morphological and histological effects of hypchlorite compounds were elucidated. However, [58] tated that avermectin could affect organs such as the fat body. The fat body of larvae and adult Diptera has specific functions regarding to the period of insect developmental stage. In this sense, it is the site of protein synthesis for the haemolymph in larvae and adults. Avermectin acting in the hyperolization of the fat boy cell membranes inhibit lipid, carbohydrate and protein uptake making with that these cytoplasmic inclusions become minors. The data from the histological studies suggest that the reduced fat body tissues may be responsible for the drastic changes observed in the biochemical profiling of hypochlorite treated larvae.
CONCLUSION
The present study aimed to determine the effect of two hypochlorite preparation, sodium and calcium hypochlorite on the four larval stages of Culex Pipiens under laboratory conditions through evaluation of : (1) Susceptibility of different larval instars to calcium and sodium hypochlorite. (2) Effect of sublethal concentrations of sodium and calcium hypochlorite on egg hatching, development, morphological changes induced by exposure of larvae to sublethal concentrations of sodium and calcium hypochlorite.
To evaluate the effect of sodium and calcium hypochlorite against Culex Pipiens larval stages, the concentration-mortality curves were constructed and the respective LC50 values were deduced. The results obtained showed that these compounds had different effects on the larval stages. Mortality rates of Culex Pipiens larvae varied according to the concentration of the hypochlorite compound. Calcium hypochlorite was more effective than sodium hypochlorite in killing 1st, 2nd, 3rd and 4th instar larvae. Also, the LC90 values indicated that calcium hypochlorite was more toxic than sodium hypochlorite in killing 1st, 2nd, 3rd and 4th instar larvae.
The present work investigated the effect of sublethal concentrations of sodium and calcium hypochlorite on Culex Pipiens larval survival rates, based on biological parameter.
Larvae that were treated with sublethal concentrations of sodium and calcium hypochlorite differed from control larvae not only in their size, but also with regard to their integument, which was very fragile and ruptured easily than observed for any of the controls. The siphon of most treated larvae was wider. All larval instars showed black patches in the paddle. The patches appeared thicker and intense in larvae exposed to LC50 of hypochlorite. The treatment of Culex pipiens larvae with sublethal concentrations of sodium and calcium hypochlorite caused degeneration of the cell structure of the alimentary canal. The mid gut cells appeared thinner and flattened, with disappearance of the peritrophic matrix. Treated larvae showed reduced fat body tissues. Muscle change was also evident. The cuticle of the body wall was thinner than that of the control. In control larvae, the Malpighian tubules were formed by cells with evident spherical nucleus and excretory products in the lumen. In larvae exposed to sublethal concentrations of hypochlorite accumulation of any substances in its lumen was not observed, which was narrow.
ACKNOWLEDGEMENT
The corresponding author is thankful to all authors about their technical support for their respective universities, research institutes and valuable help in completing this investigative project. This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (D-106-150-1441). The authors, therefore, gratefully acknowledge DSR technical and financial support.
REFERENCES
- Shahen M, Guo Z, Shar AH, Ebaid R, Tao Q, et al .(2018) Dengue virus causes changes of MicroRNA-genes regulatory network revealing potential targets for antiviral drugs. BMC Syst Biol 12: 2.
- Shahen M, Shar AH, Abd Abomohra EF, Guo Z, Wang Y (2018) Recent trends in systems biology of miRNAs and RNAi in dengue fever: Diagnosis and treatment. International Journal of Applied Research in Veterinary Medicine16.
- Shu Z, Shahen M, Hegazi M, Al-Sharkaw I, Seif A (2018) Physiological response of Culex pipiens larvae to sublethal concentrations of sodium and calcium hypochlorite. Journal of Environmental Biology 39: 314-323.
- Dyab AK, Galal LA, Mahmoud AE, Mokhtar Y (2016) Finding Walachia in filarial larvae and culicidae mosquitoes in upper Egypt governorate. Korean J Parasitol 54: 265-272.
- Gad A, Shoukry A, El-Said S (1988) Vector competence to Wuchereriabancrofti in Culex pipiens collected from the Nile Delta. J Egypt Soc Parasitol 18: 259-272.
- Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Elaziz KMM, et al. (9515) Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. The Lancet 367: 992-999.
- Dyab AK, Galal LA, Mahmoud Ael S, Mokhtar Y (2015) Xenomonitoring of different filarial nematodes using single and multiplex PCR in mosquitoes from Assiut Governorate, Egypt. Korean J Parasitol 53: 77-83.
- El E, Nagwa A (2001) Infection by certain arboviruses among workers potentially at risk of infection. J Egypt Public Health Assoc 76: 169-182.
- Turell MJ, Morrill JC, Rossi CA, Gad AM, Cope SE, et al. (2002) Isolation of west nile and sindbis viruses from mosquitoes collected in the nile valley of Egypt during an outbreak of rift valley fever. J Med Entomol 39: 248-250.
- Carapeta S, do Bem B, McGuinness J, Esteves A, Abecasis A, et al. (2015) Negeviruses found in multiple species of mosquitoes from southern Portugal: Isolation, genetic diversity, and replication in insect cell culture. Virology 483: 318-328.
- Samina I, Margalit J, Peleg J (1986) Isolation of viruses from mosquitoes of the Negev, Israel. Trans R Soc Trop Med Hyg 80: 471-472.
- Barrera R, Amador M, Clark GG (2004) The use of household bleach to control Aedes aegypti. Journal of the American Mosquito Control Association 20: 444-448.
- Ault S (1993) Environmental management: A re-emerging vector control strategy. Am J Trop Med Hyg 50: 35-49.
- De Barjac H, Sutherland DJ (2012) Bacterial control of mosquitoes & black flies: Biochemistry, genetics & applications of bacillus thuringiensis israelensis and bacillus sphaericus. Springer Science & Business Media, Netherlands.
- Lacey LA, Undeen AH (1986) Microbial control of black flies and mosquitoes. Annual review of entomology 31: 265-296.
- Olano VA, Matiz MI, Lenhart A, Cabezas L, Vargas SL, et al. (2015) Schools as potential risk sites for vector-borne disease transmission: Mosquito vectors in rural schools in two municipalities in Colombia. J Am Mosq Control Assoc 31: 212-222.
- Self L, Shin H, Kim K, Lee K, Chow C, et al. (1973) Ecological studies on Culex tritaeniorhynchus as a vector of Japanese encephalitis. Bull World Health Organ 49: 41-47.
- Randriamaherijaona S, Velonirina HJ, Boyer S (2016) Susceptibility status of Anopheles arabiensis (Diptera: Culicidae) commonly used as biological materials for evaluations of malaria vector control tools in Madagascar. Malar J 15: 338.
- Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, et al. (2010) Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J 9: 1.
- Ma K, Li X, Hu H, Zhou D, Sun Y, et al. (2017) Pyrethroid-resistance is modulated by miR-92a by targeting CpCPR4 in Culex Pipiens Comp Biochem Physiol B Biochem Mol Biol 203: 20-24.
- Tang ZH, Wood RJ, Cammack SL (1990) Acetylcholinesterase activity in organophosphorus and carbamate resistant and susceptible strains of the Culex pipiens Pesticide Biochemistry and Physiology 37: 192-199.
- Al-Sharkawi I (1997) Chlorinated lime pockets: A model proposed for the control of Schistosoma mansonitransmission in irrigation streams and ditches in Egypt. Journal-Egyptian German Society of Zoology 23: 209-236.
- Rutala W, Weber D (1999) Infection control: The role of disinfection and sterilization. Journal of Hospital Infection 43: 43-55.
- Sherman C, Fernandez EA, Chan AS, Lozano RC, Leontsini E, et al. (1998) La Untadita: A procedure for maintaining washbasins and drums free of Aedes aegypti based on modification of existing practices. The American journal of tropical medicine and hygiene 58: 257-262.
- Ritchie SA (2001) Efficacy of Australian quarantine procedures against the mosquito Aedes aegypti. J Am Mosq Control Assoc 17: 114-117.
- Di Domenico D, Ruggeri L, Trentini M (2006) The use of sodium hypochlorite as ovicide against Aedes albopictus. Journal of the American Mosquito Control Association 22: 346-348.
- Holland LZ, Kene M, Williams NA, Holland ND (1997) Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): The metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development 124: 1723-1732.
- Moriarty F (1969) The sublethal effects of synthetic insecticides on insects. Biological reviews 44: 321-356.
- Nadda G, Saxena PN, Srivastava G (2005) Effects of beta-cyfluthrin on white and sepia mutants of Drosophila melanogaster. J Environ Biol 26: 363-367.
- Selvi M, Cavas T, Benli CKA, Kocak Memmi B, Cinkilic N, et al. (2013) Sublethal toxicity of esbiothrin relationship with total antioxidant status and in vivo genotoxicity assessment in fish (Cyprinus carpio L., 1758) using the micronucleus test and comet assay. Environ Toxicol 28: 644-651.
- Antonio GE, Sanchez D, Williams T, Marina CF (2009) Paradoxical effects of sublethal exposure to the naturally derived insecticide spinosad in the dengue vector mosquito, Aedes aegypti. Pest Manag Sci 65: 323-326.
- Firstenberg D, Sutherland D (1981) Reproductive effects in Aedes aegypti following sub-lethal treatment with methoprene or Abate. NJ Mosq Control Assoc Meet 41: 111-117.
- De Coursey JD, Webster A (1953) Effect of insecticides and other substances on oviposition by aëdessollicitans. Journal of Economic Entomology 45: 1030-1034.
- Giusti A, Lagadic L, Barsi A, Thome JP, Joaquim-Justo C, et al. (2014) Investigating apical adverse effects of four endocrine active substances in the freshwater gastropod Lymnaeastagnalis. Sci Total Environ 493: 147-155.
- Wijeyaratne P (1976) Ph. D. dissertation. Univ of Texas Health Science Center at Houston; 1976. Effects of sublethal larvicide exposure on population and flight characteristic of Culex Pipiens
- Kelada N, Gaaboub I, Rawash I (1981) The effect on reproduction and morphometrics of females of Culex pipiens of treatment of larvae with six insect growth regulators. The Journal of Agricultural Science 96: 611-618.
- Belinato TA, Valle D (2015) The impact of selection with diflubenzuron, a chitin synthesis inhibitor, on the fitness of two Brazilian aedes aegypti field populations. PLoS One 10: 0130719.
- Liu W, Todd R, Gerberg E (1986) Effect of three pyrethroids on blood feeding and fecundity of Aedes aegypti. Journal of the American Mosquito Control Association 2: 310-313.
- Harbach RE (1988) The mosquitoes of the subgenus culex in Southwestern Asia and Egypt (Diptera: Culicidae). Contributions of the American Entomological Institute 24: 240.
- WHO (1981) Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. World Health Organization, Geneva, Switzerland.
- Finney DJ (1962) Probit analysis; a statistical treatment of the signoidrespoce curve. Cambridge University Press, UK.
- Hunt R, Coetzee M (1986) Field sampling of Anopheles mosquitos for correlated cytogenic, electrophoretic and morphological studies. Bull World Health Organ 64: 897-900.
- Macfie J (1915) Chlorine as a Larvicide 1915. Report. Accra Laboratory for the Year.
- Fernandez E, Leontsini E, Sherman C, Chan A, Reyes C, et al. (1998) Trial of a community-based intervention to decrease infestation of Aedes aegypti mosquitoes in cement washbasins in El Progreso, Honduras. Acta Trop70: 171-183.
- Green L, Dorough H (1968) House fly age as a factor in their response to certain carbamates. J Econ Entomol 61: 88-90.
- Hazelton GA, Lang C (1983) Glutathione S-transferase activities in the yellow-fever mosquito [Aedes aegypti (Louisville)] during growth and aging. Biochem J 210: 281-287.
- Tetreau G, Chandor-Proust A, Faucon F, Stalinski R, Akhouayri I, et al. (2014) UV light and urban pollution: bad cocktail for mosquitoes? Aquat Toxicol 146: 52-60.
- Rowland M, Hemingway J (1987) Changes in malathion resistance with age in Anopheles stephensi from Pakistan. Pesticide Biochemistry and Physiology 28: 239-247.
- Smith E, Wagenknecht A (1958) The occurrence of cholinesterase in insect eggs and its role in the ovicidal action of organophosphates. Proc Xth Int Congr Ent 2: 29-39.
- Visciarelli EC, Chopa CS, Picollo MI, Ferrero AA (2011) Cholinesterase activity during embryonic development in the blood-feeding bug Triatoma patagonica. Med Vet Entomol 25: 297-301.
- Zhang LJ, Jing YP, Li XH, Li CW, Bourguet D, et al. (2015) Temperature-sensitive fitness cost of insecticide resistance in Chinese populations of the diamondback moth Plutellaxylostella. Mol Ecol 24: 1611-1627.
- Holland PWS, Claudio D, Pwh H (1993) Essential developmental biology: A practical approach. Oxford, New York, USA.
- Landi S, Gargani E, Paoli F, Simoni S, Roversi PF (2015) Morphological markers for cryopreservation in the embryonic development of drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 108: 1875-1883.
- Han Q, Li G, Li J (2000) Purification and characterization of chorion peroxidase from Aedes aegypti eggs. Arch Biochem Biophys 378: 107-115.
- Li JS, Li J (2005) Characterization of N-linked oligosaccharides in chorion peroxidase of Aedes aegypti mosquito. Protein Sci 14: 2370-2386.
- Murugan K, Babu R, Jeyabalan D, Kumar NS, Sivaramakrishnan S (1996) Antipupational effect of neem oil and neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston). Journal of Entomological Research 20: 137-139.
- Silapanuntakul S, Keanjoom R, Pandii W, Boonchuen S, Sombatsiri K (2016) Efficacy of thai neem oil against aedes aegypti (L.) Larvae. Southeast Asian J Trop Med Public Health 47: 410-415.
- Strong L, Brown T (1987) Avermectins in insect control and biology: a review. Bulletin of entomological research 77: 357-389.
Citation: Shahen M, El-Wahsh HM, RamadanCH, Hegazi MAM, Al-Sharkawi IM, et al. (2020) A Comparison of the Toxicity of Calcium and Sodium Hypochlorite against Culex Pipiens (Diptera: Culicidae) Larvae. J Environ Sci Curr Res 3: 021.
Copyright: © 2020 Shahen M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

