
A Simple and Rapid Test for Screening Returned Fentanyl Patches in Community Pharmacies
*Corresponding Author(s):
Andrew Rey-McIntyreShoppers Drug Mart #1331, Pembroke, ON, Canada
Tel:+1 6137358682,
Fax:+1 6137357127
Email:areymcin@shoppersdrugmart.ca
Abstract
Objectives: Absence of a rapid screening test for detecting counterfeit fentanyl patches at point of care is facilitating in diversion and abuse of transdermal fentanyl. Our recent research project has made this test available and accessible to community pharmacists.
Methods: Fentanyl test strips designed to detect fentanyl in urine samples have been utilized to detect remaining fentanyl extracted from used fentanyl patches in minutes at point of care in various community pharmacies in Ontario. This method is designed to support “Safeguarding Our Communities Act” to minimize harm by preventing diversion of transdermal fentanyl.
Results: The total number of used transdermal fentanyl patches returned to 10 community pharmacies in 9 cities in Ontario during the 8-month period between April and December 2024 is 1339. 26.9% of the returned fentanyl patches were suspected counterfeit by this test. Qualitative dilution studies confirmed high sensitivity of the method.
Conclusion: Implementation of this novel screening test in every community pharmacy in Ontario is expected to minimize diversion and abuse of fentanyl patches. Moreover, availability of this test should encourage regulatory authorities to modify Patch for Patch policy guidelines and empower healthcare practitioners to make clinical decisions in a timely manner.
Introduction
Abuse and misuse of transdermal fentanyl patches leading to serious complications and fatalities is well documented [1-7]. Diversion of unused and used transdermal fentanyl patches is practiced by some individuals who abuse them in various ways including chewing8, ingestion [9], smoking [10], and intravenous injection [11,12]. Theoretically, after 72 hours of using the fentanyl patch, more than 50% of fentanyl remains. In one study [13] on cancer patients, the remaining fentanyl after a 72 hour period was found to be as high as 84%. In 2016, the government of Ontario introduced a legislation titled “Safeguarding our Communities Act” with the Patch for Patch (P4P) Return Policy guidelines (S.O. 2015). The P4P program involves the patient returning used fentanyl patches before being able to acquire new ones. The pharmacist must visually check the returned patches. If there is any suspicion that the returned patches are counterfeit, the pharmacist hands over the suspected patches to law enforcement for further investigation.
There are currently no rapid screening tests available to community pharmacists that determine whether the returned patches are counterfeit [14]. Malicious actors in Ontario have taken advantage of the absence of a screening test at community pharmacies and have started producing/and selling counterfeit patches to patients who exchange them with new patches that are diverted to the illegal market to be sold and abused. These patches are often visually difficult to distinguish from legitimate commercially available product [15]. In this research project, community pharmacies in 10 locations in Ontario participated in utilizing our rapid screening method to distinguish between authentic and counterfeit returned fentanyl patches.
Methods
Two kinds of fentanyl test-strips designed to detect fentanyl in urine were used for detection of fentanyl released from returned used transdermal patches in distilled water including:
- Fentanyl drug test kit, TN Scientific, Knoxville, USA. This kit was used in Golden Lake location
- FaStep fentanyl test strips, Trimedic, Concord, Ontario, Canada. This kit was used in all other locations in Ontario. These strips are approved by Health Canada for detecting fentanyl in liquids
When a batch of used fentanyl patches is returned to the pharmacy via the P4P program, the pharmacist inspects visually all returned patches then randomly selects a patch and removes it from the sheet by tweezers, and transfers it to a small bottle containing 5 ml of distilled water. The bottle is shaken for 2 minutes. By holding the fentanyl test strip vertically, it is dipped in the bottle for 20 seconds and then removed and left on a non-absorbable surface. The strip is read within 3 minutes.
A coloured line on the control zone indicates an authentic fentanyl patch and coloured lines on both the control and test zones indicate a counterfeit patch. If a coloured line shows on the test zone only, the test is repeated and if the coloured line continues to show on the test zone, a new kit is used. If a negative result is detected, the rest of the returned patches of the batch are handed over to law enforcement for further investigation.
Qualitative dilution studies
Dilutions by 10 folds in distilled water were used initially to test the sensitivity of the FaStep fentanyl test strips then dilutions were narrowed to 2 folds for testing Teva fentanyl patches (12, 25, 50 mcg/h).
How do fentanyl strips work?
The fentanyl strip consists of:
- Sample pad which collects the sample and begins the process of the competitive lateral flow immunoassay
- Conjugate pad containing labelled anti-fentanyl antibody
- Test zone nitrocellulose membrane containing BSA-fentanyl conjugate
- Control zone nitrocellulose membrane contains unlabelled anti-mouse immunoglobulin antibody
- Absorbent pad at the end of the fentanyl strip to absorb excessive sample and thus allowing larger sample volumes to be used to improve sensitivity and accuracy
When testing an authentic used fentanyl patch, fentanyl is released into distilled water and moves through the sample pad to combine with the labelled anti-fentanyl antibody at the conjugate zone. When reaching the test zone, it cannot combine with the BSA-fentanyl conjugate and thus no coloured line is formed at the test zone. The fentanyl combined with labelled anti-fentanyl antibody keeps moving through the nitrocellulose membrane and combines with the unlabelled anti-mouse antibody at the control zone forming a coloured line. Thus, one coloured line at the control zone demonstrates the presence of fentanyl in the tested sample and indicates the presence of fentanyl in the patch.
When testing a counterfeit patch, there is no fentanyl to be released into distilled water. Labelled anti-fentanyl antibody moves from the conjugate pad to combine with the BSA-fentanyl conjugate at the test zone forming a coloured line and uncombined labelled anti-fentanyl antibody continues moving until reaching the control zone and combines with the unlabelled anti-mouse immunoglobulin antibody forming another coloured line. Thus, when testing a counterfeit patch, a coloured line is observed at both the test and control zones.
Selection of participating locations
Invitations were sent in April 2024 to various locations in Ontario to participate in the research project. Positive replies were received from community pharmacies in Golden Lake, Pembroke (2 sites), Petawawa, Arnprior, Stittsville, Timmins, Sudbury, Midland and Ottawa. Details of the method and documentation forms were sent to all participants. The manager of each participating community pharmacy with or without another staff pharmacist was/were involved in the testing of the patches.
Analysis of data
Microsoft Excel was used to tabulate data received from participants and data were organized in Figures and Tables.
Results
Various dilutions of methanol plus water were used for extracting fentanyl from the used patches including 5+0, 4+1, 3+2, 2+3, 1+4 and 0+5 ml respectively. A clear coloured line on the control zone was observed with methanol + water at dilutions 2+3, 1+4 and 0+5 ml. A faint coloured line appeared with 3+2 ml and no coloured lines observed with 5+0 and 4+1 ml. Since distilled water alone was effective for fentanyl extraction from used patches and to avoid any risk of methanol exposure, 5 ml of distilled water was used for all subsequent testing. A new unused Teva Fentanyl 12 mcg/h was initially tested to serve as a positive control giving a coloured line at the control zone only (Figure 1). Distilled water without a patch was tested as a negative control giving coloured lines at both the test and control zones (Figure 2). Distilled water (5ml) was tested with every batch as a negative control. Figure 2 shows results of testing one of the batches demonstrating a Sandoz authentic 25 mcg/h patch, 2 different strengths (25 & 50 mcg/h) of suspected Teva counterfeit patches and distilled water as a negative control.
As demonstrated in table 1, a total of 1339 used fentanyl patches were received by community pharmacists in various locations in Ontario. Most returned patches were authentic (73.1%) while 26.9% were suspected counterfeit. Moreover, various strengths of both Teva and Sandoz brands were reported including 12, 25, 50, 75 and 100 mcg/h. The total random patches tested were 118 representing approximately 8.8% of the returned batches and confirming 979 and 360 authentic and suspected counterfeits respectively. All of returned Sandoz fentanyl patches were authentic while all suspected counterfeit patches belong to Teva brand. One patch is selected for testing not all patches of each batch are tested to provide evidence to law enforcement. Rest of untested suspected counterfeit patches were handed over to local Ontario police, with report files to be utilized for further investigation.
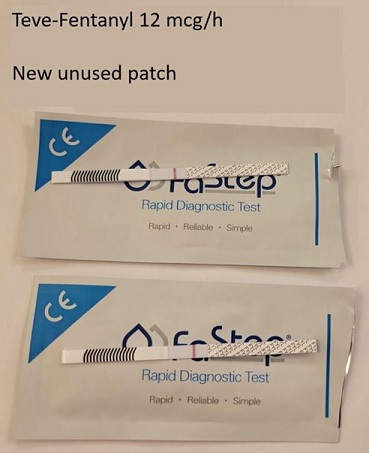 Figure 1: Images of testing a new unused 12 mcg/h Teva fentanyl patch.
Figure 1: Images of testing a new unused 12 mcg/h Teva fentanyl patch.
 Figure 2: Images of testing used returned authentic (Sandoz) and counterfeit (Teva) fentanyl patches compared to distilled water as a negative control.
Figure 2: Images of testing used returned authentic (Sandoz) and counterfeit (Teva) fentanyl patches compared to distilled water as a negative control.
|
Teva fentanyl patches |
Total patches returned |
Random patches tested per batch |
Authentic patches |
Suspected counterfeit patches |
|
12 mcg/h |
109 |
16 |
49 |
60 |
|
25 mcg/h |
250 |
23 |
235 |
15 |
|
50 mcg/h |
529 |
35 |
244 |
285 |
|
75 mcg/h |
15 |
1 |
15 |
0 |
|
100 mcg/h |
31 |
4 |
31 |
0 |
|
Sandoz fentanyl patches |
|
|
|
|
|
12 mcg/h |
35 |
3 |
35 |
0 |
|
25 mcg/h |
277 |
26 |
277 |
0 |
|
50 mcg/h |
67 |
6 |
67 |
0 |
|
75 mcg/h |
15 |
2 |
15 |
0 |
|
100 mcg/h |
11 |
2 |
11 |
0 |
|
Total |
1339 |
118 |
979 |
360 |
|
% |
100 |
8.8 |
73.1 |
26.9 |
Table 1: Classification of tested fentanyl patches returned to community pharmacies in various locations in Ontario.
As shown in table 2, one community pharmacy per city was involved in testing except in Pembroke, 2 sites were involved.
|
Location |
Total number of returned patches |
Authentic patches N (%) |
Suspected counterfeit patches N (%) |
|
Arnprior |
42 |
42 (100%) |
0 (0%) |
|
Golden Lake |
120 |
120 (100%) |
0 (0%) |
|
Pembroke (2 sites) |
407 |
62 (15.2%) |
345 (84.8%) |
|
Midland |
115 |
115 (100%) |
0 (0%) |
|
Petawawa |
160 |
145 (90.6%) |
15 (9.4%) |
|
Stittsville |
75 |
75 (100%) |
0 (0%) |
|
Sudbury |
366 |
366 (100%) |
0 (0%) |
|
Timmins |
20 |
20 (100%) |
0 (0%) |
|
Ottawa |
34 |
34 (100%) |
0 (0%) |
|
Total (%) |
1339 |
979 (73.1%) |
360 (26.9%) |
Table 2: Distribution of returned authentic and suspected counterfeit fentanyl patches in various locations in Ontario
Randomly 4 returned used fentanyl patches were selected representing 12, 25 and 50 mcg/h strengths. Initially multiple of 1:10 dilutions were used for the 12 mcg/h fentanyl patch. As demonstrated in figure 3, the highest dilution for detecting fentanyl was 1:10 which is equivalent to a volume of 50 ml distilled water.
 Figure 3: Approximate qualitative sensitivity of fentanyl strips for used Teva 12 mcg/h fentanyl patch.
Figure 3: Approximate qualitative sensitivity of fentanyl strips for used Teva 12 mcg/h fentanyl patch.
Dilution was narrowed to be multiple of 2. Figure 4 shows fentanyl is detected at 1:40 for another 12 mcg/h used patch.
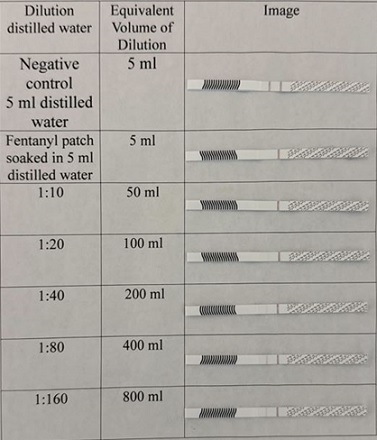 Figure 4: Approximate qualitative sensitivity of fentanyl strips for used Teva 12 mcg/h fentanyl patch.
Figure 4: Approximate qualitative sensitivity of fentanyl strips for used Teva 12 mcg/h fentanyl patch.
As demonstrated in figure 5, released fentanyl is detected at 1:640 dilution for 25 mcg/h used patch. Moreover, released fentanyl is detected at 1:320 dilution for 50 mcg/h patch (Figure 6).
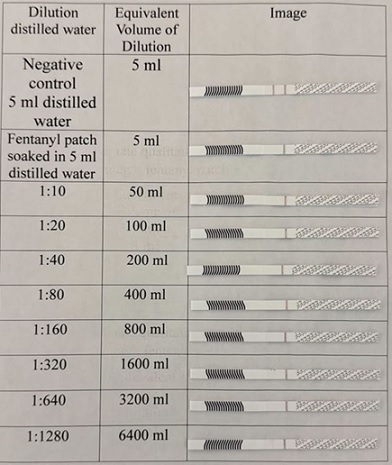 Figure 5: Approximate qualitative sensitivity of fentanyl strips for used Teva 25 mcg/h fentanyl patch.
Figure 5: Approximate qualitative sensitivity of fentanyl strips for used Teva 25 mcg/h fentanyl patch.
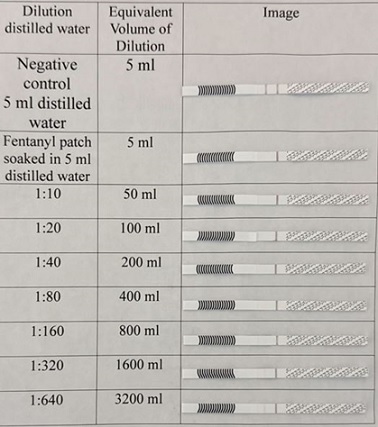 Figure 6: Approximate qualitative sensitivity of fentanyl strips for used Teva 50 mcg/h fentanyl patch.
Figure 6: Approximate qualitative sensitivity of fentanyl strips for used Teva 50 mcg/h fentanyl patch.
Discussion
Diversion of transdermal fentanyl patches is commonly practiced in Canada and may be associated with significant health, legal and social consequences [7]. Realizing the serious implications of such diversion, the government of Ontario implemented in 2016 “Safeguarding our Communities Act” P4P policy for community pharmacies which significantly reduced dispensing of fentanyl patches in some locations in Ontario [16]. Not all patients on transdermal fentanyl patches were happy with the P4P policy [17]. Malicious actors were aware that community pharmacists have no objective screening test at the point of care to confirm the returned patches are authentic. Moreover, the malicious actors have taken advantage of this inability by introducing counterfeit fentanyl patches to exchange them with authentic patches in an effort to continue diversion of authentic patches [15].
As responsible healthcare providers, our group of community pharmacists have decided to take the challenge and introduce a novel, simple and rapid test to detect any suspected returned counterfeit fentanyl patches and report the incidents to legal authorities. Our screening method in various locations in Ontario detected both authentic and suspected counterfeit fentanyl patches. Interestingly, all suspected counterfeit patches belong to Teva brand and none to Sandoz brand. This may indicate the unavailability of counterfeit Sandoz fentanyl patches in the street so far.
As per manufacturer of the fentanyl strips, the accuracy for detecting fentanyl in urine samples of patients is approximately 98%. False positive or negative results may be attributed to chemicals and metabolites in urine samples. The accuracy in testing fentanyl patches soaked in distilled water is expected to be higher due to absence of interfering chemicals and metabolites. Moreover, fentanyl strips detect nano grams of fentanyl in urine while fentanyl released from used patches is in micro grams leading to high sensitivity and possibly absence of false negatives.
Qualitative dilution testing shows released fentanyl is detected up to a dilution of 1:640 which is equivalent to 3.2 litres of distilled water. These findings confirm a relatively high sensitivity of the method and making false negative results unlikely. The results demonstrate the sensitivity to 25 mcg/h is double that of the 50 mcg/h patch. This could be attributed to the variability of fentanyl remaining after 72 hour use and may range from 28% to 84.4% [13].
Albeit the majority of the returned used fentanyl patches were authentic, widespread implementation of our testing method all over Ontario is expected to uncover a higher percentage of counterfeit fentanyl patches. Implementation of our testing method by community pharmacists is expected to minimize diversion of fentanyl patches and consequently saves the lives of victims in our community. P4P policy is aimed to minimize diversion of fentanyl patches and to prevent harm. Its existing guidelines need change by empowering pharmacists and physicians to act quickly and decisively. Any patient returning counterfeit fentanyl patches should be discovered immediately, and an individualized care plan developed by the patients’ physician and pharmacist, without unnecessary delays.
We hope regulatory authorities consider these suggestions and implement changes to P4P policy guidelines taking into consideration the pharmacist can now easily and immediately decide returned used fentanyl patches are authentic or suspected counterfeit by utilizing our rapid testing method. Moreover, our novel screening test may be utilized by law enforcement at crime scenes. The availability of this simple and rapid test may encourage pharmacy jurisdictions in other Canadian provinces to initiate and implement the suggested new P4P guidelines.
Conclusion
Community pharmacists can easily and quickly detect suspected returned counterfeit fentanyl patches by using our simple screening test.
Contributions of First Main
Andrew Rey-McIntyre initiated this research project and drafted the list of relevant studies, designed and conducted the research project, communicated with all locations in Ontario, supervised the research project, communicated with OPP and Health Canada, paid all financial costs, and performed data analysis, wrote the research manuscript.
Contributions of Second Main Author
Saafan Al-Safi collaborated with Andrew Rey-McIntyre in initiating this research project, drafting the relevant studies, designing and conducting the research project, performing data analysis and writing the research manuscript.
Contribution of Co-authors
All listed co-authors performed the testing of the returned fentanyl patches and documented the results as designed by the main authors.
Disclosure of Conflicting Interests
All authors have no financial or other conflict of interest related to this project.
Funding
This project was not funded by any commercial company or organization and the cost of fentanyl strips were paid by Andrew Rey-McIntyre/Rey-McIntyre Pharmacy Ltd.
Consents
All co-authors provided written signed consents to be included in the published research manuscript.
Disclaimer
The ideas expressed in this article are those of the authors and do not necessarily represent the views or policy of the Ontario College of Pharmacists or the Government of Canada.
Acknowledgement
We are most grateful to Professor Michael Beazely at Waterloo University for his great feedback and advice. Thanks to Mrs. Jenni Pinkerton at OATC in Pembroke, Mr. Sean MacEachern in Stittsville and Mr. Hussein Saafan in Mississauga for their help and feedback.
References
- Geile J, Maas A, Kraemer M, Doberentz E, Madea B (2019) Fatal misuse of transdermal fentanyl patches. Forensic Sci Int 302: 109858.
- Nara A, Yamada C, Saka K, Kodama T, Yoshida M, et al. (2019) A fatal case of poisoning with fentanyl transdermal patches in Japan. J Forensic Sci 64: 1936-1942.
- Bakovic M, Nestic M, Mayer D (2015) Death by band-aid: fatal misuse of transdermal fentanyl patch. Int J Legal Med 129: 1247-1252.
- Coopman V, Cordonnier J, Pien K, Van Varenbergh D (2007) LC-MS/MS analysis of fentanyl and norfentanyl in a fatality due to application of multiple Durogesic transdermal therapeutic systems. Forensic Sci Int 169: 223-227.
- Manetti F, David MC, Gariglio S, Consalvo F, Padovano M, et al. (2022) Atypical Fentanyl Transdermal Patch Consumption and Fatalities: Case Report and Literature Review. Toxics 11: 46.
- Peeters LEJ, Vleut IT, Tan GE, Croes EA, Bethlehem C (2022) Case report on postmortem fentanyl measurement after overdose with more than 67 fentanyl patches. Forensic Toxicol 40: 199-203.
- Fischer B, Vojtila L, Rehm J (2018) The 'fentanyl epidemic' in Canada - Some cautionary observations focusing on opioid-related mortality. Prev Med 107: 109-113.
- Dale E, Ashby F, Seelam K (2009) Report of a patient chewing fentanyl patches who was titrated onto methadone. BMJ Case Rep 2009: bcr01.
- Sommerfeld-Klatta K, Jiers W, Lukasik-Glebocka M, Tezyk A, Dolinska-Kaczmarek K, et al. (2023) Severe and fatal fentanyl poisonings from transdermal systems after on-skin and ingestion application. Toxics 11: 872.
- Chapman E, Leipsic J, Satkunam N, Churg A (2012) Pulmonary alveolar proteinosis as a reaction to fentanyl patch smoke. Chest 141: 1321-23.
- Reeves MD, Ginifer CJ (2002) Fatal intravenous misuse of transdermal fentanyl. Med J Aust 177: 552-553.
- Tharp AM, Winecker RE, Winston DC (2004) Fatal intravenous fentanyl abuse: Four cases involving extraction of fentanyl from transdermal patches. Am J Forensic Med Pathol 25: 178-181.
- Marquardt KA, Tharratt RS, Musallam NA (1995) Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacoth 29: 969-971.
- Ontario College of Pharmacists (2016) Patch-For-Patch Fentanyl Return Program: Fact Sheet. Ontario College of Pharmacists, Toronto, Canada.
- CBC (2017) Fentanyl patch forgeries fooling Ontario's pharmacists. CBC, Toronto, Canada.
- Tadrous M, Greaves S, Martins D, Nadeem K, Singh S, et al. (2019) Evaluation of the fentanyl patch-for-patch program in Ontario, Canada. Int J Drug Policy 66: 82-86.
- Chaudhry A (2017) Ontario patch-for-patch fentanyl return program: 6-month review of patient returns and opinions. Can Pharm J (Ott) 151: 12.
Citation: Rey-McIntyre A, Al-Safi S, Denniston T, Brown J, Falcioni B, et al. (2025) A Simple and Rapid Test for Screening Returned Fentanyl Patches in Community Pharmacies. HSOA J Addict Addict Disord 12: 200.
Copyright: © 2025 Andrew Rey-McIntyre, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

