
Antimicrobial activity of borassus flabellifer l. Root
*Corresponding Author(s):
Vincent Raja AnthoniSenior Lecturer, Department Of Mathematics, Physics And Statistics, Faculty Of Natural Sciences, University Of Guyana, Guyana
Email:vincent.anthonisamy@uog.edu.gy
Abstract
Background: Human pathogenic microorganisms have developed resistance in response to the indiscriminate use of commercial antimicrobial drugs commonly used for the treatment of infectious diseases . Borassus flabellifer belongs to the family of Arecaceae, commonly known as Palmyra palm is a native of tropical Africa but cultivated and naturalized throughout India. Different parts of the Borassus flabellifer are being used for medicinal properties . Aim: The present study was aimed to evaluate the antimicrobial activity by using separate alcoholic, petroleum ether and aqueous extract from the traditional medicinal plant, Borassus flabellifer root against different bacterial strains. Method: The antimicrobial activity of Borassus flabellifer root was performed by standard agar well diffusion methods. We selected Gram-positive bacteria such as Staphylococcus aureus, Bacillus subtilis and Gram-negative bacteria such as Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli.
Results: All the extract from root of Borassus flabellifer showed the good percentage inhibition on tested by the agar well diffusion method. The minimal bactericidal concentration was found to be at 30mg/300µl from root of Borassus flabellifer when compared with the standard drug. All bacteria were tested and showed significant antibacterial properties. Conclusion: The result of the present study concluded that the root extract of Borassus flabellifer embrace good antibacterial property. Hence it can be used for the treatment of various infectious diseases and wound healing. Furthermore, a comprehensive and systematic approach to identifying compounds for this process is required.
Keywords
Antimicrobial Activity; Borassus Flabellifer; Bacillus Subtilis; Escherichia Coli; Pseudomonas Aeruginosa; Staphylococcus Aureus; Salmonella Typhimurium.
Introduction
Medicinal plants contribute to ensuring a good source of a variety of medicines. Most people in the world rely on traditional medicine, which contains plant extracts or their active ingredients [1]. Drugs derived from plants are relatively safer than synthetic alternatives, and offer profound therapeutic benefits and affordable prices to treat a variety of ailments [2]. Antimicrobial compounds have attracted the attention of many pharmacologists and are now being part of the key areas of research. It is well known that plants with medicinal properties are rich in sources of these antimicrobial compounds [3]. In recent years, various strategies have been endorsed to overcome the resistance of antibiotics. One of the recommended strategies to achieve this goal involves the combination of other molecules with failed antibiotics, which restore the desired antibacterial activity [4]. Human pathogens have developed resistance in response to the indiscriminate use of commercial antimicrobial drugs commonly practiced in the treatment of infectious diseases [5]. With this overview, our current research work attempts to evaluate antimicrobial activity and therapeutic potential of Borassus flabellifer root for human benefit. The specie Borassus flabellifer belongs to the family of Arecaceae, different parts from this plant have been reported to comprise pharmacological functions, including anthelmintic, antidiuretic, antioxidant, antimalarial and immune modulatory activities [6], and also used as medicine for diverse illnesses in traditional medicine [7]. Male flowers are used for anti-inflammatory activity, the juice from flowering stalks used for diabetes [8]. However, there is very little systematic evidence on the biological functions of the root of this plant. Therefore, we selected root of Borassus flabellifer to evaluate antimicrobial activity in different human pathogenic bacterial strains.
Materials and Methods
Chemicals and Reagents
All the chemicals and reagents in this study were of Laboratory grade purchased from Southern India Scientific Corporation, Chennai and implemented without further purification. The standard drug Ampicillin was acquired from Apollo pharmacy, Chennai.
Collection of Plant Materials
Root products of Borassus flabellifer were collected and brought from Cuddalore district in Tamil Nadu, India. The plant materials were authenticated based on organoleptic, macroscopic examination and certified by Professor P.Jayaraman, Department of Botany, Institute of Herbal Botany, (Authenticated no: PARC/2019/4092), Plant Anatomy Research Centre, Tambaram, Chennai, India.
Preparation of Ethanolic, Petroleum Ether and Aqueous Extract Separately
Extracts were prepared as described by the standard method [9]. Initially the collected plants materials were allowed to dry in sunshade so that the muddy portions were removed. Roots of Borassus flabellifer were chopped and cut into small bites. Dried clean Borassus flabellifer roots were coarsely powdered. Approximately 30gm of plant powder was weighed and soaked with ethanol, petroleum ether and distilled water separately. Three beakers were allowed to stand for overnight or for 72 hours with intermediate shaking. Soaked solutions were passed through filter paper. The filtrate was extracted by using a Soxhlet apparatus. The extracts were concentrated to dryness by keeping them over boiling water bath. The last traces of solvent were removed by transferring them into a china dish and allow them to heat through a sand bath at normal temperature. In order to prevent charring or denature of compound, care should be taken and avoid overheating [10, 2]. The yield of the ethanolic (2.5gm), petroleum ether (2gm) and aqueous (2gm) extracts were noted for future reference. Dried crude extract is stored in sterile amber color bottles in the refrigerator until used for further investigation.
Microorganisms
The microorganism of Gram-positive strain Staphylococcus aureus and Bacillus subtilis as well as Gram negative strain such as, Pseudomonas aeruginosa, Salmonella typhimurium and Escherichia coli, were acquired as stock culture from Microbiology Lab of SRM Medical College and Research Institute, Kattankulathur, were used for the evaluation of antibacterial studies.
Aseptic Condition
Laminar flow unit is sophisticated appliance that can further help to prevent contamination of reagents and biological cultures. The laminar air flow chamber was sterilized with 90% ethanol and irradiated with short wave UV light (from laminar) for 15minutes. All other equipment used to continue the experiment was sterilized. In order to determine their viability of microorganisms, they were allowed to sub cultured in the nutrient agar medium. The identity of each test organism was confirmed by using standard culture, morphological and biochemical techniques as described [11]. Stock cultures were maintained on nutrient agar slants at 4ºC and then sub-cultured in nutrient broth at 37ºC prior to each antimicrobial test. Inoculants of the test organisms were standardized by methods [12]. This was done by suspending 5 colonies of a 24hours culture in 5ml of nutrient broth and comparing the turbidity with that of 0.5 Mac farina standards after incubating at 35ºC for 2hours[13].
Antimicrobial Assay
Preparation of Bacterial Culture Broth
Bacterial culture broth was prepared according to the standard method which contained peptone-5gm, yeast extract-3gm, Nacl-5gm, distilled water-1000ml; pH-7.0. According to the standard method, required amount was weighed, suspended in 200ml distilled water in 500ml conical flask. The media is then stirred and then autoclaved at 121±1°C for 15minutes. Then the broth was kept overnight for sterile condition and intermediately check. Next day if the media is sterile, a loop full of isolated bacterial culture were inoculated and kept for incubation at 37±1°C for 24hours.
Agar well diffusion method for bacteria:
Antibacterial studies were carried out by using the agar well diffusion method. After inoculation the agar nutrient medium (HI-Media) was prepared and transferred to 6” Petri-dishes (200mm) allowed to solidification to make base layers. Agar well diffusion method followed in order to identify the antimicrobial activity. Muller Hinton Agar (MHA) plates were swabbed (sterile cotton swabs) with 8hours old - broth culture of the respective bacteria. The seed layers were prepared by inoculating 10ml of test organism suspension in 100ml Mueller-Hinton agar (for bacteria). Wells of 5 to 6mm in diameter and about 2cm a part was made in the agar medium of each of these plates using a sterilized cork or steel borer. Dry crude petroleum ether, ethanol and aqueous extracts from Borassus flabellifer root were used as antimicrobial compounds against test microorganisms (Gram +ve and Gram -ve bacteria) 37±1°C for 24hours in the upright position. The stock solution was prepared by dissolving 1000mg of each dry crude extracts from Borassus flabellifer root in 10ml of dimethyl sulfoxide (DMSO) at a concentration of 100mg/ml separately and filtered. From the stock solution 5mg/50µl, 10mg/100µl, 15mg/150µl, 20mg/200µl, 25mg/250µl and 30mg/300µl of the concentration were added to the appropriate well by using sterile syringe. The extracts were allowed to diffuse at room temperature for 2hours without disturbed. Control (DMSO) for the experiments comprising inoculums without plant extracts were set up. Ampicillin (20µg/ml) and solvent was act as positive control. After diffusion the plates were incubated at 37±1°C for 18-24hours for bacterial pathogens. At the end of the incubation period, the plates were examined for extracts antibacterial activity by measuring the zone of inhibitions. The antibacterial zone was measured in millimeters (mm) using a measuring scale around the well of the Petri plate. The triplets were maintained. The antibacterial test was repeated three times. Each copy of the measurements was taken in three different fixed directions and the mean values were recorded. The standard error of the mean (SEM) can be calculated using the following equation: sx = S/√N Based on the SEM, the following are confidence intervals at different confidence levels. Depending on the field of study, a confidence level of 95% (or statistical significance of 5%) is typically used for data representation.
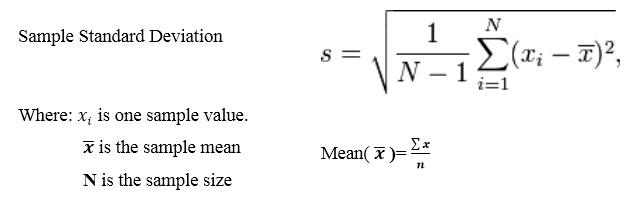
Results And Discussion
|
Zones of inhibitions of different extracts from root of Borassus flabellifer for Gram-positive and Gram-negative bacteria (mm) |
||||||
|
Name of the extract |
Concentration of extracts at (mg/µl) |
Name of the Microorganisms |
||||
|
(Stap) |
(Bas) |
(Pseu) |
(Salm) |
(Eco) |
||
|
Ethanolic extract |
Control (20µl) |
09.7±0.7 |
08.7±0.7 |
09.0±1.1 |
08.7±0.7 |
08.7±0.7 |
|
5mg / 50µl |
11.0±0.5 |
09.7±0.7 |
09.7±0.7 |
11.0±0.5 |
09.0±1.1 |
|
|
10mg / 100µl |
12.0±0.5 |
12.0±0.5 |
12.0±0.5 |
12.0±0.5 |
09.7±0.7 |
|
|
15mg / 150µl |
12.0±0.5 |
13.0±0.5 |
13.0±1.1 |
13.0±0.5 |
10.0±0.5 |
|
|
Control (20µl) |
10.0±0.5 |
09.0±1.1 |
09.7±0.7 |
09.0±1.1 |
09.0±1.1 |
|
|
20mg / 200µl |
13.0±0.5 |
14.0±0.5 |
13.0±0.5 |
14.0±0.5 |
10.0±0.5 |
|
|
25mg / 250µl |
13.0±0.5 |
15.0±0.5 |
15.0±0.5 |
15.0±0.5 |
11.0±0.5 |
|
|
30mg / 300µl |
14.0±0.5 |
18.0±0.5 |
17.0±0.5 |
16.0±0.5 |
12.0±0.5 |
|
|
Petroleum ether extract |
Control (20µl) |
- |
- |
- |
08.7±0.7 |
- |
|
5mg / 50µl |
- |
- |
- |
09.7±07 |
07.7±1.3 |
|
|
10mg / 100µl |
- |
- |
- |
10.3±0.7 |
08.3±0.7 |
|
|
15mg / 150µl |
- |
- |
- |
11.0±0.5 |
12.0±0.5 |
|
|
Control (20µl) |
10.7±0.5 |
10.7±0.7 |
10.3±0.7 |
11.5±0.5 |
09.7±0.7 |
|
|
20mg / 200µl |
11.0±0.5 |
12.0±0.5 |
13.0±0.5 |
12.0±0.7 |
13.0±0.5 |
|
|
25mg / 250µl |
13.0±0.5 |
14.0±0.5 |
14.0±0.5 |
13.0±0.5 |
14.0±0.5 |
|
|
30mg / 300µl |
14.0±0.5 |
15.0±0.5 |
15.0±0.5 |
14.0±0.5 |
15.0±0.7 |
|
|
Aqueous ether extract |
Control (20µl) |
09.7±0.7 |
- |
- |
- |
- |
|
5mg / 50µl |
10.0±0.7 |
- |
- |
10.0±0.7 |
- |
|
|
10mg / 100µl |
11.0±0.7 |
- |
13.0±0.7 |
12.0±0.5 |
- |
|
|
15mg / 150µl |
11.0±0.7 |
- |
14.0±1.3 |
13.0±1.7 |
- |
|
|
Control (20µl) |
11.0±0.7 |
09.7±07 |
13.0±0.7 |
12.0±0.7 |
08.7±07 |
|
|
20mg / 200µl |
12.0±0.7 |
10.0±0.7 |
15.0±0.5 |
14.0±0.5 |
12.0±0.5 |
|
|
25mg / 250µl |
13.0±0.5 |
11.0±0.7 |
16.0±0.7 |
15.0±0.7 |
13.0±0.5 |
|
|
30mg / 300µl |
14.0±0.7 |
12.0±3.3 |
17.0±0.7 |
16.0±0.7 |
14.0±0.7 |
|
Table 1: Antibacterial activity of Ethanolic extract from root of Borassus flabellifer for Gram-positive and Gram-negative bacteria.
Staphylococcus aureus (Stap), Bacillus subtilis (Bas), Pseudomonas aeruginosa (Pseu), Salmonella typhimurium (Salm), Escherichia coli (Eco) and Ampicillin (Amp).
The Minimal inhibitory concentration of ethanolic extract (Figure 1 (a-e), petroleum ether extracts (Figure 2 (a-e), aqueous extracts (Figure 3 (a-e), from root of Borassus flabellifer of Gram-positive and Gram-negative bacteria.
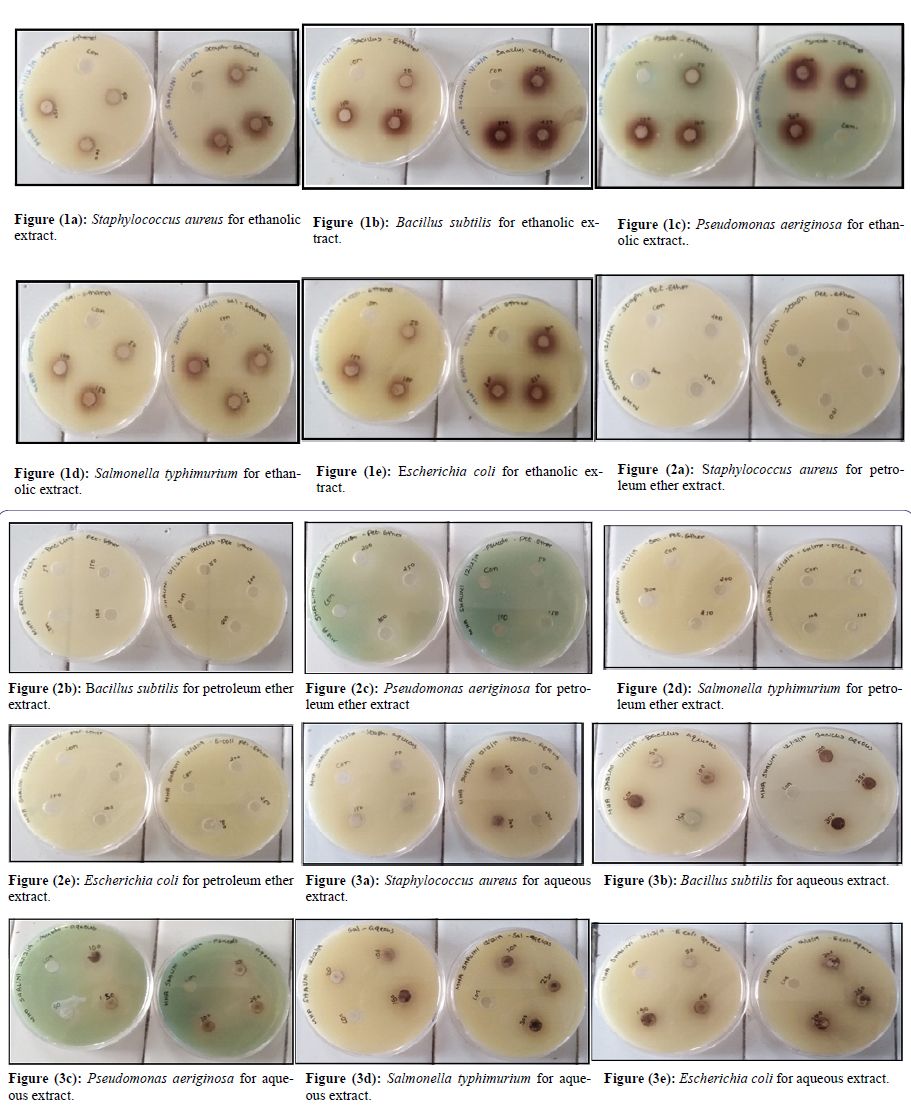 The table 1 showed different zone of inhibitions at different concentration for ethanolic, petroleum ether and aqueous extracts from the root of Borassus flabellifer against different bacterial strains such as Gram-positive (Staphylococcus aureus, Bacillus subtilis) and Gram-negative (Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) bacteria. The figure 1. (a-e) shows the zones of inhibitions were measured for each concentration on each bacterial strain in mm. The zone of inhibition for Staphylococcus aureus shows 11mm at 50µl, 12mm at 100µl, 12mm at 150µl, 13mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 9.7-10mm. The zone of inhibition for Bacillus subtilis shows 9.7mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 18mm at 300µl when compare with control that is 8.7-9mm. The zone of inhibition for Pseudomonas aeruginosa shows 9.7mm at 50µl, 12mm at 100µl, 13mm at 150µl, 13mm at 200µl, 15mm at 250µl, and 17mm at 300µl when compare with control that is 9-9.7mm. The zone of inhibition for Salmonella typhimurium shows 11mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 16mm at 300µl when compare with control that is 8.7-9mm. The zone of inhibition for Escherichia coli shows 9mm at 50µl, 9.7mm at 100µl, 10mm at 150µl, 10mm at 200µl, 11mm at 250µl and 12mm at 300µl, when compare with control that is 8.7-9mm for ethanolic extract from root of Borassus flabellifer. Earlier report said that seed coat extract of the Borassus flabellifer has been reported to possess antimicrobial activity [14], antioxidant, anthelmintic, antidiuretic, immune modulatory, and antimalarial activities [15]. The figure 2. (a-e) shows the zone of inhibition for Staphylococcus aureus shows 11mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 10.7mm. The zone of inhibition for Bacillus subtilis shows 12mm at 200µl, 14mm at 250µl, and 15mm at 300µl when compare with control that is 10.7mm. The zone of inhibition for Pseudomonas aeruginosa shows 13mm at 200µl, 14mm at 250µl, and 15mm at 300µl when compare with control that is 10.3mm. The zone of inhibition for Salmonella typhimurium shows 9.7mm at 50µl, 10.3mm at 100µl, 11mm at 150µl, 12mm at 200µl, 13mm at 250µl, 14mm at 300µl when compare with control that is 8.7-11.5mm. The zone of inhibition for Escherichia coli shows 7.7mm at 50µl, 8.3mm at 100µl, 12mm at 150µl, 13mm at 200µl, 14mm at 250µl and 15mm at 300µl, when compare with control that is 9.7mm for petroleum ether extract from root of Borassus flabellifer. Early report said that root extract of Borassus flabellifer has showed the maximum zone of inhibition (14mm) of the root extract was observed against Klebsiella pneumonia [16]. Seed coat extract of the Borassus flabellifer has been reported to possess antimicrobial activity [17]. The figure 3. (a-e) shows the zone of inhibition for Staphylococcus aureus shows 10mm at 50µl, 11mm at 100µl, 11mm at 150µl, 12mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 9.7-11mm. The zone of inhibition for Bacillus subtilis shows 10mm at 200µl, 11mm at 250µl, and 12mm at 300µl when compare with control that is 9.7mm. The zone of inhibition for Pseudomonas aeruginosa shows 13mm at 100µl, 14mm at 150µl, 15mm at 200µl, 16mm at 250µl, and 17mm at 300µl when compare with control that is 13mm. The zone of inhibition for Salmonella typhimurium shows 10mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 16mm at 300µl when compare with control that is 12mm. The zone of inhibition for Escherichia coli shows 12mm at 200µl, 13mm at 250µ, and 14mm at 300µl, when compare with control that is 8.7mm for aqueous extract from root of Borassus flabellifer. Early report said that anti-microbial activity of ethanolic, petroleum ether and aqueous extract from root of Borassus flabellifer by Agar Well Diffusion method for Gram positive and Gram-negative bacteria [13]. From this present study the minimal bactericidal concentration of ethanolic, petroleum ether and aqueous extracts from root of Borassus flabellifer for Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and Gram-negative bacteria (Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) were found to be 300μl. Therefore, the results of the present study shows that bacterial growth was inhibited at 30mg/300μl.
The table 1 showed different zone of inhibitions at different concentration for ethanolic, petroleum ether and aqueous extracts from the root of Borassus flabellifer against different bacterial strains such as Gram-positive (Staphylococcus aureus, Bacillus subtilis) and Gram-negative (Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) bacteria. The figure 1. (a-e) shows the zones of inhibitions were measured for each concentration on each bacterial strain in mm. The zone of inhibition for Staphylococcus aureus shows 11mm at 50µl, 12mm at 100µl, 12mm at 150µl, 13mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 9.7-10mm. The zone of inhibition for Bacillus subtilis shows 9.7mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 18mm at 300µl when compare with control that is 8.7-9mm. The zone of inhibition for Pseudomonas aeruginosa shows 9.7mm at 50µl, 12mm at 100µl, 13mm at 150µl, 13mm at 200µl, 15mm at 250µl, and 17mm at 300µl when compare with control that is 9-9.7mm. The zone of inhibition for Salmonella typhimurium shows 11mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 16mm at 300µl when compare with control that is 8.7-9mm. The zone of inhibition for Escherichia coli shows 9mm at 50µl, 9.7mm at 100µl, 10mm at 150µl, 10mm at 200µl, 11mm at 250µl and 12mm at 300µl, when compare with control that is 8.7-9mm for ethanolic extract from root of Borassus flabellifer. Earlier report said that seed coat extract of the Borassus flabellifer has been reported to possess antimicrobial activity [14], antioxidant, anthelmintic, antidiuretic, immune modulatory, and antimalarial activities [15]. The figure 2. (a-e) shows the zone of inhibition for Staphylococcus aureus shows 11mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 10.7mm. The zone of inhibition for Bacillus subtilis shows 12mm at 200µl, 14mm at 250µl, and 15mm at 300µl when compare with control that is 10.7mm. The zone of inhibition for Pseudomonas aeruginosa shows 13mm at 200µl, 14mm at 250µl, and 15mm at 300µl when compare with control that is 10.3mm. The zone of inhibition for Salmonella typhimurium shows 9.7mm at 50µl, 10.3mm at 100µl, 11mm at 150µl, 12mm at 200µl, 13mm at 250µl, 14mm at 300µl when compare with control that is 8.7-11.5mm. The zone of inhibition for Escherichia coli shows 7.7mm at 50µl, 8.3mm at 100µl, 12mm at 150µl, 13mm at 200µl, 14mm at 250µl and 15mm at 300µl, when compare with control that is 9.7mm for petroleum ether extract from root of Borassus flabellifer. Early report said that root extract of Borassus flabellifer has showed the maximum zone of inhibition (14mm) of the root extract was observed against Klebsiella pneumonia [16]. Seed coat extract of the Borassus flabellifer has been reported to possess antimicrobial activity [17]. The figure 3. (a-e) shows the zone of inhibition for Staphylococcus aureus shows 10mm at 50µl, 11mm at 100µl, 11mm at 150µl, 12mm at 200µl, 13mm at 250µl, and 14mm at 300µl when compare with control that is 9.7-11mm. The zone of inhibition for Bacillus subtilis shows 10mm at 200µl, 11mm at 250µl, and 12mm at 300µl when compare with control that is 9.7mm. The zone of inhibition for Pseudomonas aeruginosa shows 13mm at 100µl, 14mm at 150µl, 15mm at 200µl, 16mm at 250µl, and 17mm at 300µl when compare with control that is 13mm. The zone of inhibition for Salmonella typhimurium shows 10mm at 50µl, 12mm at 100µl, 13mm at 150µl, 14mm at 200µl, 15mm at 250µl, and 16mm at 300µl when compare with control that is 12mm. The zone of inhibition for Escherichia coli shows 12mm at 200µl, 13mm at 250µ, and 14mm at 300µl, when compare with control that is 8.7mm for aqueous extract from root of Borassus flabellifer. Early report said that anti-microbial activity of ethanolic, petroleum ether and aqueous extract from root of Borassus flabellifer by Agar Well Diffusion method for Gram positive and Gram-negative bacteria [13]. From this present study the minimal bactericidal concentration of ethanolic, petroleum ether and aqueous extracts from root of Borassus flabellifer for Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and Gram-negative bacteria (Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) were found to be 300μl. Therefore, the results of the present study shows that bacterial growth was inhibited at 30mg/300μl.
Conclusion
Information reported in this study indicated that all the extractives did not show any inhibition or mild inhibitions at lower concentrations (50µl, 100µl and 150µl) but, when the concentration was increased (200µl, 250µl and 300µl) it inhibits the growth of most of the bacterial strains tested when compared with Ampicillin which leads to formation of the zone. Screening for antimicrobial activity shows that the root extract of Borassus flabellifer indicates a potential source of new anti-infective agents. Hence it is conformed that Borassus flabellifer comprise good antibacterial property and supports its medicinal importance. It was therefore concluded that many chemotherapeutic compounds and drugs could be harvested from the root of Borassus flabellifer for many diseases. Further inquiries are being conducted to isolate the active principle compounds to sup port the notion that traditional use in medicine and primarily contributes to health care.
Acknowledgment
We would like to acknowledge Dr.R.Vasudevaraj, Principal, faculty members of Department of Biochemistry and Department of Microbiology, SRM Arts and Science College, Kattankulathur, Kanchipuram, district, for providing the laboratory facilities, encouragement and support during this work.
References
- Parmar N, Rawat M (2012) Medicinal plants used as antimicrobial agents: a review. International Research Journal of Pharmacy, 3: 31-40.
- Arirudran B, Saraswathy A, Vijayalakshmi Krishnamurthy (2011) Pharmacognostic and Preliminary Phytochemical Studies on Ruellia tuberosa (Whole plant). Pharmacognosy Journal, 3: 29-36.
- Anpin Raja RD, Prakash JW, Jeeva S (2010) Antibacterial activity of some medicinal plants used by Kani tribe, southern Western Ghats, Tamil Nadu, India. In: Trivedi PC, editor. Ethnic tribes and medicinal plans. Jaipur: Pointer Publishers, 28-45.
- Brown D (2015) Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void, Nat Rev Drug Discov 14: 821-32.
- Nesbitt M, Prance G (2005) The Cultural history of plants. Taylor & Francis 173.
- Rios JL (2010) Effects of triterpenes on the immune system. JEthnopharmacol, 128: 1-14.
- Pattanaik C, Reddy CS, Dhal NK (2008) Phytomedicinal study of coastal sand dune species of Orissa.Indian J Tradit Knowl 7: 263-8.
- Varadarajan P, Rathinaswamy G, Asirvatahm D, Rangasamy D (2008) Antimicrobial properties and phytochemical constituents of Rheo discolor. Ethnobotanical Leaflet, 12: 841-845.
- Sofowara A (1993) Medicinal plants and Traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria 289.
- Gupta AP, Verma RK, Gupta MM, Sunil Kumar (1999) Estimation of Plumbagin using High Performance, Thin Layer Chromatography, J.Med Arom 21: 661-663.
- Cowan M, Steel L (1965) Preparation of the test organisms. Niger. J. Microbiol. 22: 56-60.
- Baker CN, Thomsberry CH, Ronald WH (1983) Inoculum standardization in antimicrobial susceptibility test: evaluation of the overnight agar cultures. J. Clin. Microbiol 17: 450-457.
- Kaladhar DSVGK, Siva Kishore N (2010) Antimicrobial studies, biochemical and image analysis in Mirabilis jalapa, International Journal of Pharmacy &Technology 2: 683- 693.
- Pramod HJ, Yadav AV, Raje VN, Mohite M, Wadker G (2013) Antioxidant activity of Borassus flabellifer (Linn.) fruits. Asian JPharm Tech 3: 16-9.
- Koudouvo K, Karou DS, Kokou K, Essien K, Aklikokou K, et al., (2011) An ethnobotanical study of antimalarial plants in Togo maritime region. J Ethnopharmacol 134: 183-90.
- Senthil Kumar S, Kamaraj M (2011) Antimicrobial activity of Cucumis anguria L. by agar well diffusion method. Bot Res Int 4: 41-42.
- Duddukuri GR, Sastry YN, Kaladhar DSVGK, Rao KK, Chaitanya KK (2011) Antibacterial activity of methanolic seed coat extract of Borassus flabellifer L. Int J Pharm Sci Res 2: 2435-2438.
Citation: Arirudran B, Anthoni VR , Kumar G, Anbarasu K, Shalini E (2022) Antimicrobial activity of borassus flabellifer l. Root. Biotech Res Biochem 5: 013.
Copyright: © 2022 B Arirudran, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

