
Bioengineering Supports Preantral Follicle In Vitro Growth
*Corresponding Author(s):
Chiara Di BerardinoFaculty Of Bioscience And Technology For Food, Agriculture And Environment, University Of Teramo, 64100 Teramo, Italy
Email:cdiberardino@unite.it
Abstract
Recent advances using bioengineering methods (e.g. biomaterials, 3D-printing, microfluidics tec.) has opened the possibility to study female reproductive system and reproductive diseases in a totally new way and with unpredictable perspectives. REPROductive Tissue ENgineering (REPROTEN) effort applied to ovarian tissue has been addressed to date to build up bio-compatible artificial matrixes in which embeds immature ovarian follicles to develop an implantable ovary-like tissue for restoring the reproductive endocrine control and fertility. However, fabricated tissue scaffolds with precise shape, size, geometry, porosity, and other physical and biochemical properties to meet the native ovarian environment have recently also represented a tool to redesign the in vitro Folliculogenesis (IVfol), a technique to process the large amount of immature follicle until the production of competent oocytes that can be enrolled in the standardized procedures of in vitro maturation/fertilization before embryo transfer. Even if the IVfol reproduced on 3D-PCL patterned electrospun-extracellular matrix (ECM) scaffolds were discovered to completely support ovine preantral follicle growth up to the antral stage and the simultaneous acquisition of oocyte meiotic competence thus allowing to develop a more standardized and efficient protocol, more in-depth analyses are required to investigate whether this bioengineering methods can also recapitulate folliculogenesis and oogenesis at the molecular, genetic, and epigenetic levels.
Keywords
Artificial-ovary; Growth; In vitro follicle culture; Oocyte meiotic competence; PCL-electrospun scaffold; Preantral follicle; Sheep
Premise
Assisted Reproductive Technologies (ARTs) have a consolidated role in human and animal reproductive management. While in human, they are devoted to overcome infertility or to prevent genetic transmissible diseases, in veterinary they are mainly addressed to economical purposes or biomedical research. The most standardized in vitro ARTs are oocyte maturation (IVM), fertilisation (IVF), and embryo culture (IVC), collectively termed embryo production (IVP). It is worth of note, the pioneering experiments that opened the doors to ARTs have been set up in animal models: in 1950s the first IVF live pups were in rabbits and, one decade later, in mice whereas the first human embryo was obtained only in 1978 when Louise Brown born. Initially, to overcome the complexity of reproductive events, the procedures were performed in vivo using rabbit or sheep oviducts as recipient to then moved towards oviductal cells co-culture. Gradually, the “natural systems” where replaced by chemically defined media [1].
Nowadays, despite IVP is largely applied it is still far from to be considered a mature technology. Indeed, important issues remain to be addressed such as to ameliorate the percentage of fertilized oocytes, identify predictive markers defining oocyte/embryo quality and prevent the negative epigenetic impact of ARTs [2].
In addition, the management of the folliculogenesis phase remains an unsolved issue that limit the possibility to restore reproductive endocrine control and to enhance the availability of fertilizable female gametes by taking advantage from the large untapped reserve represented by the immature oocytes. In this context, the IVfol is the most promising technology [3] expected to face either human fertility in case of premature reduction of female gamete reserve (young oncological patients and premature menopause) or animal reproduction to enhance reproductive performance of highly selected domestic mammals or to preserve biodiversity by contrasting the reduced numerical strength of mammalian endangered species.
To overcome these claims, the reproductive research sector pays great attention to the emerging cutting edge approaches such as the innovative platforms based on the use of 3D-artificial environment [4]. The rationale of 3D-technologies consists in engineering female biomimetic reproductive tissues thus generating an integrated multi-organ in vitro systems in which the different cell compartments that physiologically control female reproductive homeostasis and function may cooperate in generating the complexity of paracrine mechanism controlling the reproductive outcomes. In a certain way the new approach is a backward path to the ARTs’ origin. The 3D-technologies, that are nowadays a tools in several branches of investigative biology and medicine started to be applied to REPROTEN [5]. In particular, REPROTEN are based on the availability of tissue-specific materials, named “scaffolds” that have to be engineered to recapitulate reproductive environments. Scaffold fulfilling reproductive matrix are currently available by taking advantage of emerging fabrication techniques such as: 1.
Laser cut
Manufacturing technique, in which a continuous wave laser is used to process materials with very high precision. Templates for the cutting process can be generated by computer-aided design (CAD). Thus, the manufacturing process is highly flexible with respect to the final design of a generated sheet and the resulting 3D-scaffold.
Three and four dimensional printing
Valuable tool for fabrication of biomimetic scaffolds that exploits the additive manufacturing technique to reach a predefined shape and well-controlled spatial chemistry and geometry adding materials layer by layer. It involves the use of 3D-software to establish a model; the model is imported into slicing software, and a 3D-printer is used to print the model [6,7]. Four-dimensional printing is an advanced version to fabricate scaffold that taking advantage from smart materials mimic the dynamic nature of tissues to a very large extent.
Electrospinning
pursues a biomimetic approach in the scaffold design [8,9] by generating nano and microfibers organized mimicking the native ECM that can be easily functionalized with active biomolecules (e.g. drugs, growth factors, proteins, etc). Recently, the scaffold-based tissue engineering approach started to be applied to the fabrication of reproductive tissues. A relevant novelty in the field [10] is the fabrication of 3D-printing and electrospun constructs able to support ovarian follicles development [5,11-14] .Among these constructs, the PCL-based scaffolds are remarkable [14]. In fact, PCL is a biodegradable polymer that has been successfully used in vivo and in vitro for multifarious regenerative purposes[15-23] and nowadays, PCL-electrospun scaffold technology has also been exerted to sustain ovarian follicles growth, viability, and preservation of the fibrillary morphology of the native follicular unit, as demonstrated by Liverani and colleagues in promoting porcine follicle viability, thus supporting the feasibility study of its use to set up artificial ovaries for transplantation [12,5]; nevertheless, few findings has been provided so far on the ability of PCL-electrospun scaffolds in supporting in vitro follicle and oocyte development, besides it is therefore essential to set up efforts to explore if this bioengineering procedures may as well faithfully reproduce folliculogenesis and oogenesis at cellular, molecular and genetic levels. As previously demonstrated [12,5,24] the topology and alignment of the PCL-fibers used for this purpose could contribute to evaluate the infiltration of ovarian follicles inside the electrospun scaffolds, maximizing reproductive performance by creating an ad hoc environment that mimics the native tissue thus potentially supporting the synergistic cooperation between the germinal and somatic compartments. To corroborate this hypothesis, the electrospun fibers oriented in a patterned manner scaffolds has recently addressed to support IVfol and proved to be successful in promoting not merely the maintenance of the follicular spherical morphology, but also the growth and antral cavity development of the ovarian functional unit itself and extremely allowing an evolutionary and meiotic development of the oocyte enclosed within [25]. Remarkably, the topology of the scaffold is essential in support the simultaneously development of germinal and somatic compartment (theca and granulosa). Indeed, despite using the same polymeric solution, when PCL-Patterned scaffolds are compared to those with randomly oriented fibers (PCL-Randomic), the results showed a sudden decrease in follicular quality, which is associated with a reduced survival, growth and development of the follicles thus compromise the in vitro oogenesis. However, that is not all: the outstanding findings obtained in term of follicle/oocyte synchronous development in vitro using PCL-Patterned scaffolds is directly proportional to the ability of the PCL-Patterned cultured follicles themselves to significantly regulate the expression profile of GJA1 gene, which plays a pivotal role in the formation of communication channels between the somatic and germinal compartments during follicular development, as well as the transcriptional activation program of the CYP17A1 and CYP19A1 genes, key step points in steroid biosynthesis, compared with follicles developed under PCL-Randomic approach [25]. This preliminary results sems to confirm also a molecular targeted developed of the in vitro growth follicles under PCL-patterned 3D conditions (Figure 1).
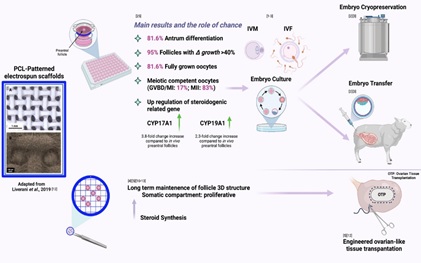 Figure 1: Bioengineering supporting IVfol overtime: main results and the role of chance – Created by BioRender.com.
Figure 1: Bioengineering supporting IVfol overtime: main results and the role of chance – Created by BioRender.com.
Conclusions
The future advancements in REPROTEN for the first time may provide great potential to generate new knowledge and technologies applied to female reproductive system to improve ARTs as well as to develop reliable in vitro models to study reproductive genetic vulnerability, to investigate the influence of several variable affecting fertility such as drugs, environmental impact and toxicities, aging, nutrition under physiological and pathological conditions.
Author Contributions
Conceptualization, B.B. and LL., methodology, B.B., L.L. and A.R.B.; formal analysis, B.B., A.R.B., L.L., C.D.B., A.P., and G.C.; investigation, C.D.B., B.B.; data curation B.B., C.D.B.; writing—original draft preparation, B.B.; writing—review and editing, C.D.B. and B.B.; visualization, B.B., L.L.; supervision, B.B.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors thank Fabiana Verni and Umberto Tosi for her valuable contribution in the retrieval and transport of the biological material.
Funding
This research was supported by the Fondo di Finanziamento di Ricerca FARDIB, University of Teramo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sjunnesson Y (2020) In vitro fertilisation in domestic mammals a brief overview Upsala Journal of Medical Sciences.125:68-76.
- van Montfoort APA, Hanssen LLP, de Sutter P, et al (2012) Assisted reproduction treatment and epigenetic inheritance.18:171-197.
- Heiligentag M, Eichenlaub-Ritter U (2018) Preantral follicle culture and oocyte quality. Reproduction Fertility and Development 30:18–43.
- Bakhshandeh B, Zarrintaj P, Oftadeh MO, et al (2017) Tissue engineering strategies tissues and biomaterials.33:144-172.
- Raffel N, Dittrich R, Bäuerle T, et al (2019) Novel approach for the assessment of ovarian follicles infiltration in polymeric electrospun patterned scaffolds.
- Bhushan B, Caspers M (2017) An overview of additive manufacturing (3D printing) for microfabrication. Microsyst Technol 23:1117-1124.
- Tamay DG, Dursun Usal T, Alagoz AS, et al (2019) 3D and 4D Printing of Polymers for Tissue Engineering Applications 7:164.
- Tan GZ, Zhou Y (2020) Electrospinning of biomimetic fibrous scaffolds for tissue engineering a review. International Journal of Polymeric Materials and Polymeric Biomaterials 69:947-960.
- Parham S, Kharazi AZ, Bakhsheshi-Rad HR, et al (2020) Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications A Comprehensive Review 13:2153.
- Gargus ES, Rogers HB, McKinnon KE, et al (2020) Engineered reproductive tissues. Nat Biomed Eng 4:381-393.
- Laronda MM, Rutz AL, Xiao S, et al (2017) A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nature Communications 8:1-10.
- Liverani L, Raffel N, Fattahi A, et al (2019) Electrospun patterned porous scaffolds for the support of ovarian follicles growth a feasibility study. Sci Rep 9:1150.
- Tamadon A, Park K-H, Kim YY, et al (2016) Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng Regen Med 13:447-454.
- Siddiqui N, Asawa S, Birru B, et al (2018) PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol Biotechnol 60:506–532.
- Kim YB, Kim GH (2015) PCL/Alginate Composite Scaffolds for Hard Tissue Engineering Fabrication, Characterization, and Cellular Activities. ACS Comb Sci 17:87-99.
- Zhu Y, Wan Y, Zhang J, et al (2014) Manufacture of layered collagen/chitosan-polycaprolactone scaffolds with biomimetic microarchitecture. Colloids and Surfaces B: Biointerfaces 113:352-360.
- Cui Z, Wright LD, Guzzo R, et al (2013) Poly( d -lactide)/poly(caprolactone) nanofiber-thermogelling chitosan gel composite scaffolds for osteochondral tissue regeneration in a rat model. Journal of Bioactive and Compatible Polymers 28:115-125.
- Firoozi N, Rezayan AH, Tabatabaei Rezaei SJ, et al (2017) Synthesis of poly(ε-caprolactone)-based polyurethane semi-interpenetrating polymer networks as scaffolds for skin tissue regeneration. International Journal of Polymeric Materials and Polymeric Biomaterials 66:805-811.
- Bolaina-Lorenzo E, Martínez-Ramos C, Monleón-Pradas M, et al (2016) Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering physicochemical characterization and Schwann cell biocompatibility. Biomed Mater 12:015008.
- Ahmed LA (2013) Stem cells and cardiac repair alternative and multifactorial approaches. J Regen Med Tissue Eng 2:8.
- Chen M-C, Sun Y-C, Chen Y-H (2013) Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomaterialia 9:5562-5572.
- Semnani D, Naghashzargar E, Hadjianfar M, et al (2017) Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. International Journal of Polymeric Materials and Polymeric Biomaterials 66:149-157.
- Zhang L, Morsi Y, Wang Y, et al (2013) Review scaffold design and stem cells for tooth regeneration. Japanese Dental Science Review 49:14-26.
- Fattahi A, Liverani L, Dittrich R, et al (2020) Optimization of Porcine Ovarian Follicle Isolation Methods for Better Developmental Potential. Tissue Eng Part A 26:712–719.
- Di Berardino C, Liverani L, Peserico A, et al (2022) When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers. Cells 11:1968.
Citation: Di Berardino C, Liverani L, Peserico A, Capacchietti G, Boccaccini AR, et al. (2022) Bioengineering Supports Preantral Follicle In Vitro Growth. J Anim Res Vet Sci 6: 029
Copyright: © 2022 Chiara Di Berardino, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

