
Bone Marrow and Blood Pictures of Broilers with BCO
*Corresponding Author(s):
Paul CotterCotter Laboratory, Arlington MA, United States
Email:kamcotter@juno.com
Abstract
The aim is to describe the hematology of broilers raised on sloped wire flooring in order to induce a high frequency of Bacterial Chondronecrosis With Osteomyelitis (BCO). Blood and bone marrow samples were obtained on d49 or d50 from both a pure strain and a cross, all were symptomatic. Samples of both tissues were made as touch preparations prepared on site and later stained with Wright-Giemsa. This method reduced the possibility that anticoagulants or storage contributed to the frequency of atypia by distortion. The total white blood counts from a portion of the samples indicated leukocytosis (>100K/μL) with atypical cells of all series. Atypical erythrocytes with irregular nuclei and anuclear forms (erythroplastids) were detected in both blood and bone marrow. Atypical and toxic granulocytes were in both tissues. In some samples bone marrow granulocyte types (meso and metamyelocytes) were found in blood. Mitotic and amitotic cells of the plasmacyte series were also in both tissues. Cells having condensed chromosomes without a spindle apparatus (aspindylosis) were also detected; a first report. Aspindylosis cells were either osteoblasts or plasmacytes. Granulated histiocytes (ggh) with giant nuclei were present in both tissues indicating polyploidy. Cell associated (CAB) and free bacteria and fungi were commonly seen in both blood and bone marrow. In some cases, the fungi expressed morphologic characteristics indicating they were examples of Hemomycetes Avium (Ha) a widely distributed organism of lame chickens, ducks, and turkeys. Oher fungi, possibly Microsporum sp. were detected as well. In summary the wire floor raised broilers readily developed BCO and were an excellent source of atypia of blood and bone marrow. The observations will contribute to a better understanding of the complexity of lameness in poultry.
Keywords
Aspindylosis; Atypia; BCO; Hematology ; Hemomycetes avium
Abbreviations
BCO: Bacterial Chondronecrosis with Osteomyelitis
CAB: Cell Associated Bacteria
CM: Cell Membrane
FNH: Femoral Head Necrosis
GGH: giant granulated histiocyte
HA: Hemomycetes Avium
H: Hyphae
H: Heterophil
H/L 1, H/L 2 : Heterophil/lymphocyte ratio
RC: Reactive Clusters
SDC: Standard Differential Count
TWBC: Total White Blood Cell Counts
Introduction
Bacterial Chondronecrosis with Osteomyelitis (BCO) also known as Femoral Head Necrosis (FHN) develops because of poor mineralization of leg bone cartilage cells followed by overgrowth of opportunistic bacteria. Both immunosuppression and stress have been implicated as factors contributing to development of BCO [1]. Because of lameness BCO in broilers is a welfare issue. Field cases have been associated with immunosuppression driven by exposure to several viruses and bacteria [2]. A useful experimental model system in which broilers grown on wire flooring readily develop BCO was introduced by Wideman [3]. The model is designed to produce BCO without a deliberate exposure of flocks to infectious agents. An alternative floor-litter/bacterial challenge method has also been developed [4]. Both techniques result in a rapid onset of BCO lesions.Multifactorial diseases as BCO where infectious agents, stress, and other causal factors are fully integrated should provide an appropriate theater for development of complex blood pictures. This is true since infections per se are expected to alter the hemogram. Stress, without infection can also be reflected in the hemogram; sometimes demonstrated by a change of the heterophil/lymphocyte ratio. However, this method is often flawed [5-7]. Since most of cells of the circulation originate in bone marrow, BCO should have profound effects on this tissue as well as blood. The purpose here is to describe atypical cells of blood and bone marrow detected during experimental BCO induced in broilers by the wire-rearing technique. Although emphasis is on leukocytes and their interactions, cells of the erythrocyte series are also considered. These observations may provide additional insight as to how animals cope with disease.
Materials And Methods
Animal procedures were approved by the University of Arkansas Animal Care and Use Committee (protocols #08036 and #11002). Samples were obtained from two trials; the first came from broilers of strain C, at day 50, and a cross of C with strain H obtained at day 49. Blood taken on site was drawn from a wing vein and immediately spread across the surface of microscope slides. Bone marrow touch preparations came from femurs obtained immediately after euthanizing. Slides were air-dried on site in a warm environment.
- Staining
All slides were post-fixed by immersion in EtOH for 15 min. staining was by Wright’s method using an in-house procedure followed by a brief exposure to Giemsa.
- Light Microscopy and Photomicrographs
Olympus CX-41(Olympus America, Center Valley, PA 18034-0610) equipped with Plan N 40x, 0.65 numerical aperture dry, and Plan N, 1.25 numerical aperture 100x oil objectives. All images were captured at 100x with an Infinity-2 1.4-megapixel charge-coupled device Universal Serial Bus 2.0 Camera, and processed with Infinity Analyze software (Release 6.5) (Lumenera, Inc., Ottawa, ON, Canada).
- Bone Marrow and Blood Cell Nomenclature
Cells of the leukocyte and erythrocyte series were named following guidance for normal adult and embryonic bone marrow as per Lucas and Jamroz [8]. Granuloblast, promyelocyte, mesomyelocyte, metamyelocyte, and mature cells compose the granulocytic series. Erythroblasts, early, mid, and late polychromatic erythrocytes, and mature (fully hemoglobinized) erythrocytes. Other cells are osteoblasts and osteoclasts, and a histiocyte form (described here). The Lymphocyte series are lymphoblasts, lymphocytes (small, Ls and medium sized/reactive, Lm) and plasmacytes, PC. Thrombocytes are indicated as needed. An asterix preceding an abbreviation used for a cell indicates it is atypical as *HC (toxic/atypical heterophil).
- Standard Differential Count and Total White Cell Counts
Two counts of 200 leukocytes/slide were sorted using criteria as described by Lucas and Jamroz [8] and in [9,10]. The designation “typical heterophil” (HT) as used here was assigned to the most frequent type seen in earlier studies. Classic heterophils (HC) resemble those most often illustrated in avian hematology literature. Rare variant heterophils (HV) are distinct from both HT and HV [9,10]. Total white blood counts (TWBC) were determined by a modified microscopic method as described in [11]. Standard Differential Counts (SDC) were determined at 40x magnification.
- H/L Ratio Calculation
Division of the sum of all three heterophil types by the small “resting” lymphocytes (Ls) gives the H/L 1; [H/L 1 = (HC + HT + HV) / Ls]. Division of the same heterophil value by the sum of all lymphocyte types, (resting Ls, medium reactive (Lm) gives the H/L 2; [H/L 2 = [HC + HT + HV) / (Ls + Lm)]. ΔH/L = H/L 1 − H/L.
Results
- Standard Differential Counts (SDC)
Average values (percent) for SDCs are in (Table 1). Due to the small sample size (N =18, Strain C; N=10 Strain C x H) and the inability to determine some total white counts (N =10, Strain C; N=4 Strain C x H) because of cell aggregates (RC, reactive clusters) statistical tests were not performed. However, average TWBCs for the pure (Strain C) samples were 107K/μL and 121K/μL for the cross (Strain C x H) values well above leukocytosis.
|
Strain |
No. |
HC1 |
HT |
HV |
Ls |
Lm |
NK |
Bst |
Mn |
Ba |
Eo |
H/L 1 |
H/L 2 |
ΔH/L |
No. |
TWBCK) |
|
C |
18 |
0.2 |
53.3 |
2.2 |
18.5 |
7.1 |
0.2 |
0.1 |
15.4 |
3.0 |
0.0 |
4.8 |
3.4 |
1.4 |
10 |
108 |
|
C x H |
10 |
0.0 |
58.1 |
2.4 |
16.9 |
9.0 |
0.2 |
0.1 |
7.4 |
5.7 |
0.1 |
12.8 |
4.4 |
8.4 |
4 |
121 |
Table 1: Standard differential counts in % from study blood samples with a limited TWBC.
- Atypical Erythrocytes and Blood Fungi
Examples of erythrocytes and leukocytes in broilers with BCO are in (Figure 1A & 1B). Background cells: mature erythrocytes (fully hemoglobinized) are in a neighborhood also containing a solitary toxic heterophil, cell 5. Cell 1 is an erythroplastid with umbilicus at its right end; likely formed at the nuclear expulsion site [12]. Cell 2 is an RBC with umbilicus (dacrocyte, arrow); likely the result of separation of a microerythroplastid like Cell fragment 8 (see also Figure 4B). Cell 3 is a small RBC, a microcyte. Cell 4 is a toxic heterophil, possibly of the HT type [9]. Cell 6 is possibly a free RBC nucleus ejected from a cell like # 1. It would be a “pyrenocyte” if it possessed a cytoplasmic rim. Many of these disintegrate and appear as “smudge cells.” All RBC nuclei appear highly condensed and several are irregularly shaped. Cell 7 is possibly a small lymphocyte (Ls). Panel B. A high cellularity blood field with Heterophils (HC) a Monocyte (Mn) a Basophil (Ba) also contains the mycelial form of a blood fungus Hemomycetes Avium (Ha) [13,14] (Figure 2).
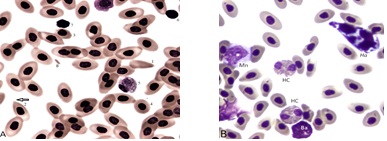 Figure 1A: A blood field with polygonal RBC.
Figure 1A: A blood field with polygonal RBC.
Panel A. Heterogeneity among blood erythrocytes of broiler strain C. The field contains normal (mature) RBCs and several atypical types and a toxic heterophil. Panel B. A blood field with polygonal RBC, classic heterophils (HC) a monocyte (Mn) a basophil (Ba) and a mycelial form of Hemomycetes avium (Ha). Additional descriptions are in the text.
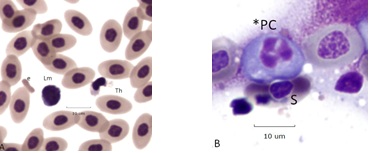 Figure2A: Tripolar Amitosis of a Plasmacyte
Figure2A: Tripolar Amitosis of a Plasmacyte
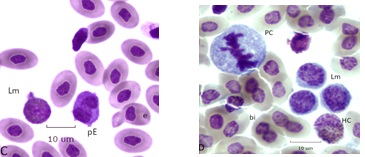 Figure 2: Dividing Cells.
Figure 2: Dividing Cells.
An aborted form of mitosis associated with BCO, described as “aspindylosis,” occurs when chromatin condenses into chromosomes in the absence of a spindle apparatus. Examples are in (Figure 3A). Panel A (bone marrow). Cells 1 and 2 are osteoblast like with giant (polyploid) nuclei. The chromatin is coarse and fused nucleoli are visible. The cytoplasm is mildly basic. The chromatin of cell 3 has condensed into individual chromosomes whose size range parallels the chicken karyotype of (~6) macro and microchromosomes. The dispersed chromosomes are at metaphase thickness without evidence of a spindle apparatus (aspindylosis). Cell 4 is a leukoplastid (anuclear fragment) with Mott-type vacuolated cytoplasm [15,16]. Cell 5, an irregular plasmacyte, is partially phagocytosed by cell 3. Panel B. An example of aspindylosis in a blood plasmacyte. The dispersed chromosomes are fully condensed but remain unattached to a spindle apparatus. A microerythroplastid is attached to the surface of a nearby RBC (arrow) (Figure 3B).
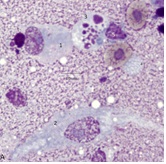 Figure 3A: Bone Marrow
Figure 3A: Bone Marrow
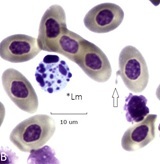 Figure 3B: Aspindylosis of a plasmacyte
Figure 3B: Aspindylosis of a plasmacyte
Cells 1 and 2 are osteoblasts, cell 3 is aspindylosis, cell 4 a leukoplastid, cell 5 a plasmacyte (bone marrow).
- Giant Granulated Histiocytes
Reactive monocytes of bone marrow are associated with BCO (Figure 4A & 4B). Cells 1 and 2 are giant (tetraploid) cells with an irregular cell membrane [17,18]. Cell 2 is at mitotic metaphase with a central chromatin mass and lagging chromosomes. One member of chromosome pair #1 in Cell 2 is in the process of being jettisoned into the cytoplasm (arrow). Cell 3 is a classic heterophil, cells 4 and 5 are small lymphocytes (Ls) with irregular cell membranes. Cells 6 are possibly thrombocytes. Cell 7 is an RBC in the process of producing an erythroplastid [12]. Cell 8 is a binuclear RBC. Panel B. A 3 cell RC composed of an early giant granulated histocyte (ggh1) [19] and a prophase stage (with large fused nucleoli (ggh2). Late stage (tetraploid) proerythrocytes and mature RBCs also appear.
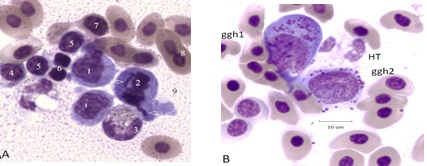 Figure 4: Panel A. Reactive and mitotic Mn cells in a mixed cluster. Panel B. Giant granulated histiocytes and polyploid erythroid cells in BCO bone marrow. Additional descriptions are in the text
Figure 4: Panel A. Reactive and mitotic Mn cells in a mixed cluster. Panel B. Giant granulated histiocytes and polyploid erythroid cells in BCO bone marrow. Additional descriptions are in the text
- Atypical Granulocytic Cells
Bone marrow types are rarely found in normal hemograms, in BCO many cells normally occupying the bone marrow are in the circulation (Figure 5 ). Panel A has a pair of giant progranulocytes (mesomyelocytes) whose nuclei are highly polyploid (> 4N). Cell 1 is an early developmental form, cell 2 has primary and specific granules and clear vacuoles indicating it is more mature. Cell 3 is a toxic HT. Panel B has a mildly toxic HC and a circulating mesomyelocyte, ordinarily a resident cell of the bone marrow. A toxic (HC) metamyelocyte of the blood is in Panel C. A giant (4+) toxic HC and a normal variant heterophil of the blood are in panel D.
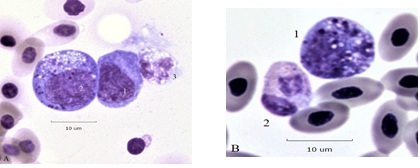 Figure 5A: Cell 1 granuloblast Cell 2 giant mesomyelocyte. Figure 5B: Cell 1 Mesomyelocyte (presumptive HC type) of blood
Figure 5A: Cell 1 granuloblast Cell 2 giant mesomyelocyte. Figure 5B: Cell 1 Mesomyelocyte (presumptive HC type) of blood
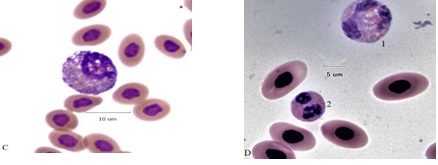 Figure 5C: Toxic (2+) giant metamyelocyte (HC-type) Figure 5D: 1 toxic (4+) giant HC, cell 2 variant heterophil (HV).
Figure 5C: Toxic (2+) giant metamyelocyte (HC-type) Figure 5D: 1 toxic (4+) giant HC, cell 2 variant heterophil (HV).
Figure 5: Heterophil granulocytic series of BCO bone marrow and blood.
Discussion
Injury caused by BCO are of interest to poultry producers because of its negative economic impact and to a larger audience because of welfare concerns. Although detection of lameness may be straight forward at the gross level. Examination of blood and bone marrow provides an additional window into its pathology. Lameness appearing in flocks grown under normal conditions would suggest existence of predisposing factors, perhaps not easily identified. Here, however, flocks were grown under conditions deliberately designed to cause lameness. Therefore, the observations on blood and bone marrow are expected to be amplifications of what may occur under more mild conditions. None-the-less they may be worthwhile to those whose interests’ extend to basic questions.
Here it has been shown that multiple types of atypical cells occur during experimental BCO. This is not a surprise. However, some of the forms of atypia have not been described elsewhere. These are a high frequency of atypical RBC as those in Figure 1A. It is often true that when atypical RBCs are found so are atypical leukocytes;
While multiple bacterial species have been isolated from lame poultry [20] it is apparent that polymicrobial interactions are likely important. The capacity of polymicrobial colonies to attach to RBC is important because it is likely that only a few of the several bacterial species comprising a colony can be isolated (Figure 6A). Do CAB strains (Figure 6A) induce hemolysis and does the release of hemoglobin contribute to BCO damage [21].
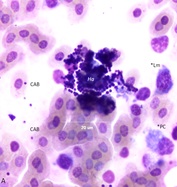 Figure 6A: Polymicrobial colony composed of bacteria and fungi (Ha) of blood.
Figure 6A: Polymicrobial colony composed of bacteria and fungi (Ha) of blood.
Fungi associated with lameness have received little attention. Hemomycetes avium [22] has been detected in blood and bone marrow from a wide variety of (lame) poultry (Figure 6A). Other fungi as Microsporum sp. (Figure 6B) are likely to be co-morbidity factors.Changes of the hemogram associated with BCO go well beyond those usually associated with stress. The presence of BM cells such as granuloblasts, progranulocytes, and toxic heterophils in blood immediately obviates the use of the standard H/L ratio as a stress determinant (Table 1) [5,18].
The appearance of plasmacytes (PC) and reactive lymphocytes [17] in the circulation or an increase of their frequency in BM should of interest to immunologists. Why are cells designed to assist in defense against bacterial disease not able to thwart BCO at an early stage? Perhaps such cells contribute to the injury. The easily recognizable Mott-type PC are a remarkable group and are seen in both BCO blood and BM [15,16]. What roles do dividing PC play in exasperation or resolution of disease? Circulating cells capable of division, by either mitosis or amitosis, are remarkable by themselves. In normal BM cell division is not common, occurring at <1% [8]. To the author’s knowledge aspindylosis, the presence of condensed chromosomes without a spindle apparatus is distinct from apoptosis (nuclear fracture) [10] and has not been reported elsewhere. This remarkable phenomenon (Figure 4A, B) should be of interest to a wide variety of investigators.
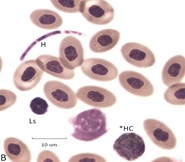 Figure 6: Examples of blood fungi associated with BCO. Panel A polymicrobial colony composed of bacteria and fungi (Ha) of blood. Panel B. Segmented hyphae (H) possibly of Microsporum gallinae in a blood field with a Ls and a toxic heterophil (*HC)
Figure 6: Examples of blood fungi associated with BCO. Panel A polymicrobial colony composed of bacteria and fungi (Ha) of blood. Panel B. Segmented hyphae (H) possibly of Microsporum gallinae in a blood field with a Ls and a toxic heterophil (*HC)
Conclusion
In conclusion, experimentally produced BCO is an effective disease model with the potential for multiple uses. The efficacy of treatments and management conditions designed to alleviate lameness an identify welfare issues are amenable to this method. Moreover, experimental BCO provides a means to investigate atypical blood and bone marrow cells because they are found in high frequency. Furthermore, as demonstrated here the experimental BCO technique is useful in the study of blood fungi.
Acknowledgment
The author thanks Robert Wideman, Jr. Ph.D., University of Arkansas, Fayetteville, AK, USA for assistance with sample collection.
Competing interests
None declared.
Ethical Consideration
Ethical issues (including plagiarism, consent to publish, misconduct, double publication and/or submission, and redundancy) have been checked by the sole author.
References
- Wideman, RF, Prisby RD (2013) Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis Front. Endocrinol (Lausanne) 3: 183.
- Bradshaw RH, Kirkden RD, Broom D (2002) A review of the aetiology and pathology of leg weakness in broilers in relation to their welfare. Avian Poult Biol Rev 13: 45-103.
- Alrubaye AAK, Ekesi NS, Hasan A, Koltes DA, Wideman RF , et al. (2020) Chondronecrosis with osteomyelitis in broilers: further defining a bacterial challenge model using standard litter flooring and protection with probiotics. Poultry Science 99: 6474-6480.
- Wideman, RF , Hamal KR, Stark JM, Blankenship J, Leste H, et al. (2012) A wire-flooring model for inducing lameness in broilers: Evaluation of probiotics as a prophylactic treatment. Poult Sci 91: 870-883.
- Cotter PF (2015) Atypical lymphocytes and leukocytes in the peripheral circulation of caged hens. Poultry Science 1439-145.
- Cotter PF (2022) Stress assessment by the hemogram method - circulating cells complicating reliance on heterophil/lymphocyte (H/L) ratio. J Vet Med Res 9: 1224.
- Cotter PF (2023) Aviary caged hens with unusual lymphocytes and normal H/L ratios. Asian Journal of Research in Animal and Veterinary Sciences 6: 115-122.
- Lucas AM, Jamroz C (1961) Atlas of Avian Hematology. US Dept of Agriculture Washington DC.
- Cotter PF, Heller ED (2016) Complex hemograms of isolator raised specific pathogen free (SPF) chicks. Int J Poultry Science 15: 211-217.
- Cotter PF (2021) Apoptosis of circulating heterophils; implications for the interpretation of the heterophil/lymphocyte ratio. J Immunological Sci 5: 1-8.
- Campbell TW, Ellis CK (2007) Avian and Exotic Animal Hematology and Cytology. Third Edition Blackwell Publishing Ames Iowa.
- Cotter PF (2021) Erythroplastids of duck blood produced by cytokinesis, lysis, and amitosis. J World Poult Res 11: 271-277.
- Cotter PF (2015) Hemomycetes avium, a blood-borne fungus of three poultry species. Abst Poultry Science.
- Cotter PF (2022) Cytogenetics of reactive bone marrow associated with a fungal infection (Hemomycetes avium) of ducklings. World J Vet Sci 4: 1018.
- Cotter PF (2015) Are peripheral Mott cells an indication of stress or inefficient immunity? Poultry Science 94: 1433-1438.
- Cotter PF, Bakst MR (2017) A comparison of Mott cell morphology of three avian species. II. – Bad behavior by plasmacytes? Poultry Science 96: 325-331.
- Cotter P, C Buckley (2018) Giant cells and plasmacyte atypia of duck blood (frank indicators of immunosuppression) Proceedings: 67th Western Poultry Disease Conference 46-47.
- Cotter PF (2023) Aviary caged hens with unusual lymphocytes and normal H/L ratios. Asian Journal of Research in Animal and Veterinary Sciences 6: 115-122.
- Cotter PF, Fetrow B (2021) Bone marrow histiocytes of lame ducklings resemble hemophagocytic histiocytes Abst Poultry Science 101(E Supplement-1).
- Bielke LR, Hargis BM, JD Latorre (2017) Impact of enteric health and mucosal permeability on skeletal health and lameness in poultry. Adv Exp Med Biol 1033: 185-197.
- Pretini V, Koenen MH, Kaestner L, Fens M, Schiffelers RM, et al. (2019) Red blood cells: chasing interactions. Front Physiol
- Cotter PF (2023) Recent observations on Hemomycetes avium a blood fungus of poultry. Abst Poultry Science 102(E Supplement-1).
Citation: Cotter P (2023) Bone Marrow and Blood Pictures of Broilers with BCO, J Anim Res Vet Sci 7: 0.48.
Copyright: © 2023 Paul Cotter, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

