
Bronchial hyperreactivity (BHR): An old but gold hallmark of asthma
*Corresponding Author(s):
Cristina BoccabellaDepartment Of Cardiovascular And Pulmonary Sciences, Università Cattolica Del Sacro Cuore, Rome, Italy
Email:cristina.boccabella@gmail.com
Abstract
Bronchial hyperreactivity (BHR), also defined as airway hyperreactivity (AHR), is still nowadays one of the noteworthy pathophysiological elements defining asthma. It is described as an excessive airway narrowing in response to different stimuli leading to increased air flow resistance and significantly decreased forced expiratory volume in 1 second (FEV1. BHR may be provoked by environmental stimuli, both chemical and physical (e.g., inhaled allergens, drugs, etc.), or physiological stimuli such as hyperventilation or physical exercise. The phenomenon of BHR involves a series of airway structural and functional changes, which can contribute to the onset of several pathological conditions, not necessarily limited to the respiratory system.
Keywords
Asthma; Bronchial hyper reactivity; Remodelling.
Introduction
The assessment of BHR is traditionally based on bronchial challenge tests, which can be classified as direct or indirect. The first relies on direct binding of bronchial smooth muscle receptors, causing airway narrowing and represents the “fixed component”’ of BHR. On the other hand, the inflammatory status (i.e., eosinophilic airway inflammation) better correlates with indirect challenges that represent the “variable component”[1-3]. To date, BHR can be considered as a pathological expression of a combination of different mechanisms [4]. The aim of this review is to summarise the state of the art about BHR, from the pathophysiology to diagnostic methods, with a particular focus on its contribution to asthma.
Epidemiology
BHR is considered as the direct expression of variable airflow which is undoubtedly one of the characteristic features of asthma. As it is virtually present in all asthmatics, its measurement by challenge tests can be a valuable tool for predicting, confirming or excluding asthma, as well as for evaluating disease progression and decline of lung function [5]. Nevertheless, BHR is not an exclusive feature of asthma as it can also be found in healthy subjects with a rate of incidence ranging from 11% to 20% [1,6]. Furthermore, BHR has been associated with a broad spectrum of diseases other than asthma. Atopic diseases represent one of the main risks of developing BHR. In patients affected by Allergic Rhinitis (AR), studies from bronchial biopsies found an association between sputum ECP levels and BHR severity [6]. Since the knowledge related to AR and BHR correlation in not fully understood, the “one airway” theory supports a deep interaction between airway and nasal dysfunction through the presence of eosinophilic inflammation in the upper and lower airway [7]. Moreover, in the “Sapaldia study” BHR has been recognized as possible risk factor for the development and progression of Chronic Obstructive Lung Disease (COPD), showing an unfavourable synergistic effect with active smoke habit [8]. Finally, it has been shown that BHR is increased in cystic fibrosis [9,10], particularly if detected through methacholine challenge test (MCT) [11], and in cardiovascular diseases such as congestive heart failure, mitral valve disease and vasospastic angina [1,12].
Pathophysiology
Mechanisms underlying BHR are not yet fully acknowledged. Different elements such as heritable components, airway inflammation, structural abnormalities and neurological abnormalities have been reported to be involved. Evidence suggests that BHR is composed of two ‘semi-independent components’, fixed or persistent BHR and episodic or variable BHR [13]. Although both are influenced by airway inflammation, they maintain specific characteristics, mostly related to different responses to several tests and treatments, resulting in a variable pathophysiological and clinical interpretation [1].
Fixed (or persistent) BHR refers to the non-responsive or partially responsive nature component of BHR to anti-inflammatory treatment (i.e., inhaled corticosteroids, environmental control). It can be considered as the expression of non-reversible airway structural changes triggered by chronic inflammation better known as “airway remodelling” [1,13]. The main airway structural changes include subendothelial basement membrane deposition of collagen (fibrosis), airway smooth muscle hypertrophy and/or hyperplasia, increased vascularity, and changes in extracellular matrix composition, which all contribute to increased airway wall thickness [14]. Airway epithelium contributes extensively to triggering airway remodelling through the release of several inflammatory cytokines such as transforming growth factor beta (TGF-β), thymic stromal lymphopoietin (TSLP), interleukin (IL) 33 and granulocytes-macrophage colony-stimulating factor (GM-CSF). Many other cytokines such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and vascular endothelial growth factors (VEGFs), as well as chemokines (e.g., CXCL2, CXCL3, IL-8/CXCL8), also contribute to airway remodelling in asthma [15]. Fixed BHR is clinically detected with direct stimulus methods, such as methacholine and histamine challenge tests, that directly interact with airway muscle receptors (M3 for methacholine and H1 for histamine). The persistent nature of fixed BHR makes it unsuitable as a measure of disease activity [4].
Variable (or transient) BHR reflects acute airway inflammation, usually but not necessarily involving eosinophils, mast cells and basophils. An atopic IgE-mediated response seems to be the most common inducer of variable BHR; however, respiratory tract infections (predominantly viral infections) [16,17] and environmental exposure (such as in occupational asthma) are also frequently recognized as variable BHR inducers. Differently from fixed BHR, the degree of variable BHR is associated with disease clinical severity and can be detected with several indirect stimuli methods, such as mannitol, adenosine monophosphate (AMP), physical exercise, hypertonic saline and hyperpnea of dry air, which induce the release of mediators from inflammatory cells [18]. Often, a correlation can be observed between the used method of testing and some biomarkers mainly involved in T2 inflammation pathway. The AMP method closely correlates with sputum and blood eosinophils and eosinophil cationic protein while the use of mannitol reflects more closely fraction of exhaled nitric oxide (FeNO) levels [19]. For these reasons, indirect challenges can be used to identify potential ICS responder patients and monitor the effectiveness of the therapy (18). Asthma and BHR are typically associated with eosinophilic inflammation in which T2 cytokines, such as IL-3, IL-4, IL-5, and IL-13 play a key role [20]. On the other hand, non-inflammatory mechanisms such as central and peripheral neurohumoral modulation can impact the bronchial tone [21]. Although evidence has highlighted the relationship between airway eosinophilic inflammation and BHR, there is no uniform consensus on this, and T2-independent inflammation is currently becoming an intriguing study field. Th-9 cells are a subset of cells which preferentially produce IL-9 when exposed to IL-4 and TGF-β. IL-9 is traditionally implicated in mast cell proliferation, goblet cell hyperplasia, IL-13 production and in inducing airway eosinophilic inflammation as well as an eosinophil-independent BHR. Furthermore, Th-9 cell-mediated BHR as well as non-T2 asthma (e.g., neutrophilic asthma) is observed to be resistant to glucocorticoid treatment [20–23].
Risk factors
Segregation analysis confirmed that a familial component exists in BHR transmission and that this is not due to a single autosomal locus [20]. Genes involved in BHR transmission are likely to be localized on chromosome 5 and coinherited with IgE genetic loci. In fact, elevated serum IgE levels strongly correlate with BHR [24]. In addition to this, cytokines involved in allergic and eosinophilic cascades, such as GMCS and IL-3, -4, -5, -9 and -13, are localized on the same region of chromosome 5q. Interestingly, atopic subjects, with or without asthma, seem to have a significantly higher incidence of BHR rather than the general population [25]. Nevertheless, it is also possible to detect BHR among non-atopic asthmatic patients. Van Herwerden et al. found a genetic linkage between FcεRIβ chain and BHR, even without a relevant linkage between atopy and the same locus, suggesting that BHR genetic transmission may be independent from atopy genes [26].
Cough variant asthma is an atypical asthma manifestation in which BHR is mildly increased; in these patients the continuative use of ICS contributes to decreasing the response to MCT and preventing the development of typical asthma, demonstrating that anti-inflammatory treatment reduces BHR even in early stages [27].
Among early risk factors, low birth weight and length seem to be associated with the development of BHR. A longitudinal prospective community-based study with follow-up at the age of 14 years has shown that the strongest risk factor for developing BHR was female sex, probably due to smaller lungs and narrower airway calibre [28,29]. Furthermore, it has been documented there may be a possible correlation between maternal and/or paternal history of asthma and symptoms suggestive of asthma in early life with the subsequent development of BHR [30].
BHR has an increased prevalence in smokers compared to the general population and it is likely associated with a dose-dependent pattern and with initiation of smoking before 20yrs of age [31]. It is uncertain whether BHR is caused by the inhalation of smoke itself, or if it is a consequence of the accumulation of smoking-induced airway injury. Nevertheless, the high prevalence in older smokers suggests that it may develop as an effect of cumulative tissue damage [32]. Following smoking cessation, the severity of BHR may improve, while a return to normality is rare [33].
Most evidence suggests that there is a correlation between BHR and obesity, especially in women [34,35]. Burgess et al. revealed an independent association between obesity and BHR surprisingly demonstrating that the association was significant only in subjects with no history of asthma. This correlation can be partly explained by small airway closure in obese participants defined as the percentage change in forced vital capacity (FVC) from baseline to the end of MCT. In fact, obesity can facilitate small airway closure because of its mechanical effect on airway diameter, particularly at low volumes [36]. Furthermore, the pro-inflammatory status associated with obesity could also affect BHR by altering pulmonary surfactant structure and function [37], increasing surface tension at the air-liquid interface of small airways and promoting an early closure [38]. Celedòn et al. also reported an increased risk of BHR among adult males in families living in rural areas [39].
Acute lower respiratory tract infections (LRTIs) seem to increase the risk of BHR development, with viral infections as the most common agents. Viral infections can induce BHR through the following major mechanisms: stimulation of bronchospastic inflammatory mediators, imbalance of the autonomic nervous system control of airway muscles and aberrant augmentation of the immune responses [40]. Regarding the first mechanism, it has been proposed that the susceptibility to viral-induced airway dysfunction is related to a high gene expression of IL-4 and IL-5 in the airways and low gene expression of IL-2 and INF-γ [24]. This pathway contributes to BHR pathogenesis such as submucosal infiltration from activated lymphocytes, eosinophils and mast cells [41]. Furthermore, viral infections cause epithelial necrosis in the lower airway, followed by epithelial desquamation that enhances BHR and allows the penetration of irritant agents or allergens [42,43]. These observations suggest the possibility that respiratory viral infections may induce acute inflammation which causes transient BHR [43,44] as well as recurrent further infections [45].
Diagnostic Tests
Airway hyperresponsiveness can be safely and reliably measured using challenge tests that expose subjects to stepwise increases in stimulus doses while performing pulmonary function tests to obtain a bronchoconstrictor dose-response curve [5].
Direct tests use pharmacological agents such as methacholine, histamine, cysteinyl leukotrienes (LTC 4, LTD 4, LTE 4) and prostaglandins (PGD2, PGF2, and thromboxane A2), which directly act on specific airway smooth muscle receptors provoking airway narrowing [46]. From these, MCT is often the first diagnostic choice. Methacholine is a synthetic derivative of acetylcholine and acts on muscarinic receptors (M3) in airway smooth muscle [47]. According to the American Thoracic Society (ATS) and the European Respiratory Society (ERS) Guidelines Task Force, during the challenge, the dose or the concentration of methacholine is progressively increased, until a fall in FEV1 of ≥20% from baseline is observed. The test is defined as diagnostic if the concentration (PC20) or the dose (PD20) that caused that reduction, is respectively equal or less than 8 mg/mL (PC20 ≤8 mg/mL) or 200 micrograms (PD20 ≤200 mcg) [48].
Histamine is an inflammatory mediator produced by basophils and mast cells acting on H1 receptors of airway smooth muscle. Histamine produces similar effects to methacholine but is less used in clinical practice, due to its frequent side effects such as flushing and headaches [5].
On the other hand, indirect challenge tests use different stimuli such as physical exercise, hypertonic saline, hyperpnoea of dry air, mannitol and adenosine monophosphate (AMP) to stimulate the release of inflammatory mediators and induce airway narrowing [6].
Thanks to its safety and its single standard protocol, the inhaled mannitol test is the most used indirect challenge test. It acts by increasing the osmolarity of the airway leading to the release of inflammatory mediators from eosinophils and mast cells. Mannitol is delivered in several steps through a dry powder inhaler in increasing doses up to a cumulative dose of 635mg [49]. The AMP challenge is mainly used in research settings due to the absence of an official agreement on the performance standard. The AMP binds to specific receptors that trigger the release of inflammatory mediators such as prostaglandins, leukotrienes, and histamine. It can be performed using the five-breath dosimeter or the 2 minutes tidal breathing protocol with increasing concentrations (up to 300–400 mg/mL) of AMP. The test is considered diagnostic when a 20% fall in FEV1 (PC20 AMP) is observed [50].
Exercise challenge testing (ECT) with treadmill, free running, or cycle ergometry, is usually adopted to reveal an exercise-induced bronchoconstriction (EIB). Even though EIB is one of the better-known predictive factors for asthma, [51], (Figure 1) it can also be present in subjects without asthma [52,53]. The ECT test is not easy to standardize because of the necessity of dispensing dry air at very high flow rates and the requirement of specialized personnel, making it difficult to use in daily clinical practice [54, 55].
 Figure 1: Exercise-Induced Bronchoconstriction (EIB) in athletes.
Figure 1: Exercise-Induced Bronchoconstriction (EIB) in athletes.
Voluntary hyperventilation (EVH) testing requires patient to breathe through a bag of dry air enriched with CO2 to avoid problems created by hypocapnia. The patient is asked to breathe forcefully for at least 6 minutes, simulating an exercise. The test allows patients to easily reach high levels of ventilation, which is particularly useful in professional athletes. However, the poor control of effort intensity, the risk of causing severe bronchoconstriction and environmental factors are the major limits of this test [56].
According to 2023 GINA guidelines, indirect bronchial tests that use standardized hyperventilation, hypertonic saline or mannitol are defined as diagnostic for BHR when a fall in FEV1≥15% from baseline is observed while ECT is diagnostic for BHR when a fall of FEV1 of 10% and 12% predicted from baseline is observed respectively for adult and children [57].
Direct and indirect bronchoprovocation tests are not interchangeable due to the different underlying mechanisms of action. Direct tests have the better negative predictive factor in excluding asthma. In contrast indirect tests are more specific in confirming the diagnosis [18].
Small airway disease (SAD) parameters have been recently tested to predict BHR [58]. As BHR and SAD are known as markers of poor asthma control, Katsoulis and co-workers suggested an equation which linked the risk of having BHR with Asthma Control Test (ACT) values and Impulse Oscillometry (IOS) parameters (i.e., X5) [59,60]. Furthermore, Short P. and colleagues found no significant differences when directly comparing the effect on IOS measurements during MCT and mannitol challenge test with FEV1 decline. For the first time these studies introduced IOS as a surrogate of FEV1 during BHR challenge tests improving safety and reproducibility [59]. Diagnostic tests are resumed in Table 1.
|
|
Direct tests |
Indirect Tests |
|
Stimuli |
Methacholine Histamine |
Mannitol Exercise Voluntary hyperventilation Hypertonic saline solution Adenosine monophosphate |
|
Mechanism
|
Binding specific airway smooth muscle receptors (M3 or H1) |
Releasing inflammatory mediators |
|
Mainly dependent from |
Smooth muscle function and airway caliber
|
Airway inflammation |
|
Dose and reproducibility |
Dose not limited, easy to perform |
Dose limited, generally more difficult to perform |
|
Characteristics |
High sensitivity |
High specificity |
|
Optimal utilization |
Exclusion of current asthma diagnosis |
Confirm current asthma diagnosis Evaluate ICS responsiveness Monitor therapy effectiveness |
|
Indications |
Methacholine: asthma diagnosis |
Mannitol: ICS responsiveness and therapy effectiveness Exercise: EIB suspected: sportsmen - children Voluntary hyperventilation: EIB suspected: athletes Hypertonic saline solution: children –induced sputum requested |
Table 1: Characteristics of direct and indirect BHR test.
BHR And Asthma
Asthma is a chronic inflammatory airway disorder in which many cells and cytokines lead to recurrent episodes of wheezing, chest tightness and coughing. BHR is often regarded as a 'hallmark' of asthma and bronchoprovocation testing is frequently performed to support the diagnosis. [61,62]. Important insights into the pathogenesis of BHR were not obtained until the last few decades introducing it as a fundamental tool to identify mild and early onset stages and furthermore to assess future risk of developing asthma. Singh et al. conducted a study in which 50 symptomatic subjects with normal spirometry underwent methacholine and exercise tests. The majority (85%) of them were found to be positive at PC20 of 16mg/ml, confirming the high negative predictive value of MCT, as well as its value in predicting the development of future clinical asthma [63]. Indeed, a two-year follow-up study in young students showed that the higher the BHR, the more likely they would develop asthma. About 45% of asymptomatic school-age subjects with a significative PD20 developed asthma in the following two years, and 80% of them had a history of early respiratory illness [64]. The presence of BHR is associated with an underlying airway remodelling process which involves airway structural changes, as already mentioned above (Figure 2). Histamine challenge test seems to be able to detect airway remodelling, particularly ASM hypertrophy which is mostly correlated with severe long-term asthma [15, 65].
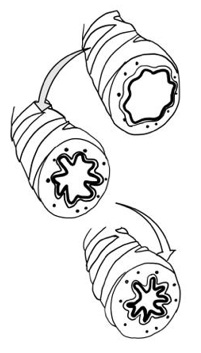 Figure 2: Airway remodeling and narrowing: The presence of BHR is associated with an underlying airway remodeling process which involves small airway structural changes such as goblet cell metaplasia, subepithelial deposition of matrix protein, fibrosis, and overexpression of angiogenic factors and hyperplasia/hypertrophy of airway smooth muscle (ASM).
Figure 2: Airway remodeling and narrowing: The presence of BHR is associated with an underlying airway remodeling process which involves small airway structural changes such as goblet cell metaplasia, subepithelial deposition of matrix protein, fibrosis, and overexpression of angiogenic factors and hyperplasia/hypertrophy of airway smooth muscle (ASM).
With their potential ability to modify the natural history of the disease, biological drugs have completely renewed the perspective of patients with severe asthma and pioneered the concept of remission. In this perspective, BHR has regained considerable interest as it could represent a treatable key-trait [66]. However, only few studies have investigated the impact of biologics on BHR and to date no approved biological therapy is clearly associated with a BHR improvement [67]. Omalizumab, a humanized anti-IgE monoclonal antibody (mAb), is an important option for treating uncontrolled allergic asthma. A randomized controlled trial (RCT) found significant improvements in the provocative dose of acetylcholine required to decrease PC20 after 16 weeks of omalizumab in moderate-to-severe allergic asthma [68]. However, in another study including patients with mild-to-moderate asthma, near-depletion of airway mucosal IgE and eosinophils on bronchial biopsy specimens was not accompanied by improvements in methacholine PC20. In mild-to-moderate asthmatics improvement in BHR was considered as a primary outcome, but omalizumab did not improve methacholine or AMP PC20 compared with placebo after 12 weeks-treatment. [69] Mepolizumab, Reslizumab, and Benralizumab are anti–eosinophilic drugs currently available for treating hyper-eosinophilic asthma. As eosinophil concentration in bronchoalveolar lavage (BAL) is greater in subjects with asthma and a positive MCT, it has been postulated that airway eosinophil suppression with the anti−IL-5 monoclonal antibody mepolizumab would contribute to BHR attenuation. Conversely, subjects with refractory asthma, did not detect any improvements in methacholine AHR after 52 weeks of high-dose intravenous 750mg mepolizumab every 4 weeks [70]. In another RCT, subjects with mild asthma did not improve BHR after 20 weeks treatment with mepolizumab and, despite blood and bronchoalveolar lavage eosinophils being mostly suppressed, 50% of airway tissue and bone marrow eosinophils remained with ongoing cell degranulation (as evidenced by persistence of major basic protein) [71]. Benralizumab, an anti−IL-5Ra monoclonal antibody, showed a significant improvement in mannitol AHR, differently from mepolizumab. The discordant results related to the two different anti-eosinophilic strategies might be explained by Benralizumab eosinophils complete depletion in contrast with the merely suppression obtained with Mepolizumab, and to the differences in airway challenges adopted [67,72]. Dupilumab is a human mAb which targets the α-subunit of the IL-4/13 receptor. It could be reasonable to hypothesise that a suppression of IL-13 inflammation would improve BHR through its effect on mucus hypersecretion and potentiation of airway narrowing. Ongoing RCTs investigating the effect on mannitol and methacholine seem to show a reduction of BHR secondary to the blockage of IL-4/13 [73]. TSLP is an alarmin of increasing scientific interest as it is expressed in the epithelium and submucosa and leads to an increase in allergic responses to the bronchial allergen challenge in asthmatics. It is also involved in all the mechanisms related to airway remodelling [15,23]. Studies testing Tezepelumab (a human IgG2-λ mAb which binds TSLP) effect on asthma, showed a reduction in BHR to mannitol in the treatment group compared to placebo. Furthermore, a significant proportion of patients in the anti-TSLP arm had a negative mannitol test at the end of treatment. It has been hypothesised that the mechanism by which Tezepelumab abrogates airway hyperresponsiveness might involve effects on mast cell activation together with the reduction in both bronchial and blood eosinophils [74,75]. Despite all these findings, the current evidence shows that we are far from understanding if BHR can really represent a treatable trait in asthmatics and if biological therapies can cover an active role in this field, especially in patients with severe disease in which structural abnormalities are more rooted [15] .
Asthma remission is an emerging concept which refers to an optimal clinical and functional control with or without ongoing treatment. So far, only heterogeneous expert opinions are available regarding this concept and all the definitions are influenced by other chronic inflammatory diseases (i.e., connective tissue diseases). Recently, an expert consensus framework has proposed a definition of asthma remission which includes a negative BHR testing as a criterion for complete remission on treatment.[66] Further studies are needed to better identify factors which could predict clinical remission with a particular focus on the role of BHR.
Conclusions and Future Perspectives
Over the last decades, BHR has been regarded as a valuable tool in supporting diagnosis and predicting asthma development. Recently, BHR has increasingly attracted new interest because of its relationship with different agents involved in inflammatory mechanisms, especially T2 inflammation, and consequently its ability to predict corticosteroid-response. BHR testing is also able to indirectly detect structural pathological changes which determine airway remodelling, typically involved in disease progression. Then, as the development of biologicals has led to a dramatic change in the asthma management, BHR is becoming even more relevant. Indeed, it seems to reveal immunomodulating activity of these drugs, their effect on restoring epithelial damage and finally their implications in terms of disease control and remission. Thus, considering all these findings, it is reasonable to reconsider the role of BHR as an actual and promising element increasing diagnostic accuracy, supporting endotyping assessment and providing an opportunity to tailor asthma treatment. Further studies are needed to fully understand its potential in advancing personalized asthma assessment.
Author contribution and conflict of interests
GM, VA, DCD, FC searched and collected literature evidence and drafted the manuscript. FC, EF, CB and MB, were involved in the critical revision of the manuscript. All authors approved the final version.
Conflict of Interest Statement. GM, VA, DCD and CB declare no conflicts of interests. FC and Doxa Pharma. CB has received personal fees from AstraZeneca, GSK, Sanofi, Menarini, Chiesi. MB has received grants/research support and honoraria or consultation fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Grifols, GSK, Menarini, Sanofi.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowlgement
We gratefully thank Federica Ficili and Giorgio Maida for the figure’s design.
References
- Borak J, Lefkowitz RY (2016) Bronchial hyperresponsiveness. Occup Med (Lond) 66: 95-105.
- Currie GP, Jackson CM, Lipworth BJ (2023) Does bronchial hyperresponsiveness in asthma matter? J Asthma 41: 247-258.
- Leuppi JD, Brannan JD, Anderson SD (2002) Bronchial provocation tests: The rationale for using inhaled mannitol as a test for airway hyperresponsiveness. Swiss Med Wkly 132: 151-158.
- Blais CM, Davis BE, Nair P, Cockcroft DW (2021) Direct and indirect bronchoprovocation tests in dose-response studies of inhaled corticosteroids: Past, present, and future directions. Allergy 76: 1679-1692.
- O’Byrne PM, Gauvreau GM, Brannan JD (2009) Provoked models of asthma: what have we learnt? Clin Exp Allergy 39: 181-192.
- Comberiati P, Katial RK, Covar RA (2018) Bronchoprovocation Testing in Asthma: An Update. Immunol Allergy Clin North Am 38: 545-571.
- Lluncor M, Barranco P, Amaya ED, Domínguez-Ortega J, López-Carrasco V, et al. (2019) Relationship between upper airway diseases, exhaled nitric oxide, and bronchial hyperresponsiveness to methacholine. J Asthma 56: 53-60.
- Zanini A, Cherubino F, Zampogna E, Croce S, Pignatti P, et al. (2015) Bronchial hyperresponsiveness, airway inflammation, and reversibility in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 10: 1155.
- Chen Q, Shen Y, Zheng J (2021) A review of cystic fibrosis: Basic and clinical aspects. Animal Model Exp Med 4: 220-232.
- Mitchell I, Corey M, Woenne R, Krastins IRB, Levison H (2023) Bronchial hyperreactivity in cystic fibrosis and asthma. J Pediatr 93: 744-748.
- Burdon JGW, Cade JF, Sutherland PW, Pain MCF (2023) Cystic fibrosis and bronchial hyperreactivity. Concomitant defects or cause and effect? Med J Aust 2: 77-78.
- Nishimura Y, Yu Y, Kotani Y, Nishiuma T, Lin S, et al. (2001) Bronchial hyperresponsiveness and exhaled nitric oxide in patients with cardiac disease. Respiration 68: 41-45.
- Cockcroft DW, Davis BE (2006) Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol 118: 551-559.
- Gillis HL, Lutchen KR (1985) Airway remodeling in asthma amplifies heterogeneities in smooth muscle shortening causing hyperresponsiveness. J Appl Physiol 86: 2001-2012.
- Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, et al. (2022) Biologics and airway remodeling in severe asthma. Allergy 77: 3538-3352.
- Johnston SL (1997) Bronchial hyperresponsiveness and cytokines in virus-induced asthma exacerbations. Clin Exp Allergy 27: 7-9.
- Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA (1976) Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis 113: 131-139.
- Anderson SD (2010) Indirect challenge tests: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138: 25S-30S.
- Park YA, Park H Bin, Kim YH, Sul IS, et al. (2017) Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide in children with asthma. J Asthma 54: 644-651.
- Boulet LP (2003) Physiopathology of Airway Hyperresponsiveness. Curr Allergy Asthma Rep 3: 166-171.
- Baroffio M, Barisione G, Crimi E, Brusasco V (2009) Noninflammatory mechanisms of airway hyper-responsiveness in bronchial asthma: An overview. Ther Adv Respir Dis 3:163-174.
- Saeki M, Nishimura T, Kitamura N, Hiroi T, Mori A, et al. (2019) Potential Mechanisms of T Cell-Mediated and Eosinophil-Independent Bronchial Hyperresponsiveness. Int J Mol Sci 20: 2980.
- Gour N, Wills-Karp M (2015) IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75: 68-78.
- Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, et al. (1995) Genetic susceptibility to asthma--bronchial hyper responsiveness coinherited with a major gene for atopy. N Engl J Med 333: 894-900.
- Fish JE, Rosenthal RR, Batra G, Menkes H, Summer W, et al. (1976) Airway responses to methacholine in allergic and nonallergic subjects. Am Rev Respir Dis 113: 579-586.
- van Herwerden L, Harrap SB, Wong ZYH, Abramson MJ, Kutin JJ, et al. (1995) Linkage of high-affinity IgE receptor gene with bronchial hyperreactivity, even in absence of atopy. Lancet 346: 1262-265.
- Fujimura M, Hara J, Myou S (2005) Change in bronchial responsiveness and cough reflex sensitivity in patients with cough variant asthma: effect of inhaled corticosteroids.
- Leynaert B, Bousquet J, Henry C, Liard R, Neukirch F (1997) Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med 156: 1413-20.
- Rijcken B, Schouten JP, Mensinga TT, Weiss ST, De Vries K, et al (1993) Factors associated with bronchial responsiveness to histamine in a population sample of adults. Am Rev Respir Dis 147: 1447-1453.
- Collins RA, Parsons F, Deverell M, Hollams EM, Holt PG, et al. (2011) Risk factors for bronchial hyperresponsiveness in teenagers differ with sex and atopic status. J Allergy Clin Immunol 128: 2.
- Juusela M, Pallasaho P, Rönmark E, Sarna S, Sovijärvi A, et al. (2013) Dose-dependent association of smoking and bronchial hyperresponsiveness. Eur Respir J 42: 1503-1512.
- Vianna EO, Gutierrez MRP, Barbieri MA, Caldeira RD, Bettiol H, et al. (2008) Respiratory effects of tobacco smoking among young adults. Am J Med Sci 336: 44-49.
- Willemse BWM, Postma DS, Timens W, ten Hacken NHT (2004) The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J 23: 464-476.
- Chinn S, Jarvis D, Burney P (2002) Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax 57: 1028-1033.
- Sood A, Dawson BK, Eid W, Eagleton LE, Henkle JQ, et al. (2005) Obesity is associated with bronchial hyper-responsiveness in women. J Asthma Jul 42: 847-852.
- Burgess JA, Matheson MC, Diao F, Johns DP, Erbas B, et al. (2017) Bronchial hyperresponsiveness and obesity in middle age: insights from an Australian cohort. Eur Respir J 50: 1602181.
- Hohlfeld JM, Schmiedl A, Erpenbeck VJ, Venge P, Krug N (2004) Eosinophil cationic protein alters pulmonary surfactant structure and function in asthma. Journal of Allergy and Clinical Immunology 113: 496-502.
- Shore SA (2010) Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol 108: 735-743.
- Celedón JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, et al. (2001) Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med 164: 1835-1840.
- Futrakul S, Deerojanawong J, Prapphal N (2005) Risk factors of bronchial hyperresponsiveness in children with wheezing-associated respiratory infection. Pediatr Pulmonol 40: 81-87.
- Djukanovic R, Roche WR, Wilson JW, Beasley CRW, Twentyman OP, et al. (1990) Mucosal inflammation in asthma. Am Rev Respir Dis 142: 434-57.
- Busse WW (1989) The relationship between viral infections and onset of allergic diseases and asthma. Clin Exp Allergy 19: 1-9.
- Eggleston PA (1988) Upper airway inflammatory diseases and bronchial hyperresponsiveness. J Allergy Clin Immunol 81: 1036-41.
- Bardin PG, Johnston SL, Pattemore PK (1992) Viruses as precipitants of asthma symptoms. II. Physiology and mechanisms. Clin Exp Allergy 22: 809-22.
- Hogg JC (1992) Persistent and latent viral infections in the pathology of asthma. Am Rev Respir Dis 145.
- Cockcroft DW (2010) Direct challenge tests: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138: 18S-24S.
- Coates AL, Wanger J, Cockcroft DW, Culver BH, Carlsen KH, et al. (2017) ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J 49.
- Hallstrand TS, Leuppi JD, Joos G, Hall GL, Carlsen KH, et al. (2018) ERS technical standard on bronchial challenge testing: pathophysiology and methodology of indirect airway challenge testing. Eur Respir J 52.
- Sverrild A, Leadbetter J, Porsbjerg C (2021) The use of the mannitol test as an outcome measure in asthma intervention studies: a review and practical recommendations. Respir Res 22.
- Ahmed S, Handa A (2021) Diagnostic value of bronchoprovocation challenge with adenosine monophosphate versus exercise testing in early diagnosis of asthma. Med J Armed Forces India 77: 46-50.
- Bonini M, Silvers W (2018) Exercise-Induced Bronchoconstriction: Background, Prevalence, and Sport Considerations. Immunol Allergy Clin North Am 38: 205-214.
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, et al. (2013) An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 187:1016-27.
- Bonini M, Lapucci G, Petrelli G, Todaro A, Pamich T, et al. (2007) Predictive value of allergy and pulmonary function tests for the diagnosis of asthma in elite athletes. Allergy 2: 1166-70.
- Randolph C (2011) Diagnostic exercise challenge testing. Curr Allergy Asthma Rep 11: 482-90.
- Ernst P, Ghezzo H, Becklake MR (2002) Risk factors for bronchial hyperresponsiveness in late childhood and early adolescence. Eur Respir J 20: 635-9.
- Rundell KW, Anderson SD, Spiering BA, Judelson DA (2004) Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest 125: 909-15.
- 2023 GINA Main Report. Global Initiative for Asthma – GINA.
- Asano T, Kanemitsu Y, Takemura M, Fukumitsu K, Kurokawa R, et al. (2020) Small airway inflammation is associated with residual airway hyperresponsiveness in Th2-high asthma. J Asthma 57: 933-41.
- Short PM, Anderson WJ, Manoharan A, Lipworth BJ (2015) Usefulness of impulse oscillometry for the assessment of airway hyperresponsiveness in mild-to-moderate adult asthma. Ann Allergy Asthma Immunol 115: 17-20.
- Katsoulis K, Kipourou M, Nicola Quaranta V, Kostikas K (2022) Predicting Bronchial Hyperresponsiveness in Patients with Asthma: The Role of Impulse Oscillometry. Arch Bronconeumol 58:722-724.
- Brannan JD, Lougheed MD (2012) Airway Hyperresponsiveness in Asthma: Mechanisms, Clinical Significance, and Treatment. Front Physiol 3: 460.
- Papi A, Brightling C, Pedersen SE, Reddel HK (2018) Asthma The Lancet. Lancet Publishing Group 391: 783-800.
- Singh S (2021) Bronchial challenge test in patients with a history suggestive of bronchial asthma with normal spirometric studies. Med J Armed Forces India 77: 82-85.
- Zhong NS, Chen RC, Yang MO, Wu ZY, Zheng JP, et al. (1992) Is asymptomatic bronchial hyperresponsiveness an indication of potential asthma? A two-year follow-up of young students with bronchial hyperresponsiveness. Chest 102: 1104-1109.
- Tsurikisawa N, Oshikata C, Tsuburai T, Saito H, Sekiya K, et al. (2010) Bronchial hyperresponsiveness to histamine correlates with airway remodelling in adults with asthma. Respir Med 104: 1271-1277.
- Menzies-Gow A, Bafadhel M, Busse WW, Casale TB, Kocks JWH, et al. (2020) An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin 145: 757-765.
- Chan R, Lipworth B (2023) Efficacy of biologic therapy on airway hyperresponsiveness in asthma. Ann Allergy Asthma Immunol 131: 37-41.
- Noga O, Hanf G, Kunkel G (2003) Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol 131: 46-52.
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. (2004) Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 170: 583-893.
- Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, et al (2009) Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 360: 973-984.
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS (2003) Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 167: 199-204.
- Chan R, RuiWen Kuo C, Jabbal S, Lipworth BJ (2023) Eosinophil depletion with benralizumab is associated with attenuated mannitol airway hyperresponsiveness in severe uncontrolled eosinophilic asthma. J Allergy Clin Immunol 151: 700-705.
- Manson ML, Säfholm J, James A, Johnsson AK, Bergman P, Al-Ameri M, et al. (2020) IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. Journal of Allergy and Clinical Immunology 145: 808-817.
- Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, et al. (2021) Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 9: 1299-1312.
- Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, et al. (2014) Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 370: 2102-2110.
Citation: Boccabella C, Cefaloni F, Adiletta V, Monteleone G, Cotugno Depalma D, et al. (2023) Bronchial hyperreactivity (BHR): An old but gold hallmark of asthma. J Allergy Disord Ther 6: 011.
Copyright: © 2023 Cefaloni F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

