
Burden of Pneumococcal Disease: An 8-Year Retrospective Analysis of Local Surveillance Data from Three Regions in Uganda (2012-2020)
*Corresponding Author(s):
Ikiriza AntonyDepartment Of Public Health, Mountains Of The Moon University, P.O.Box 837, Fort Portal, Uganda
Email:iantony2011@gmail.com
Abstract
Background
Pneumococcal disease is a leading preventable cause of childhood diseases and deaths globally with many of the deaths occurring in low and middle-income countries. In Uganda, Pneumococcal Conjugate Vaccine 13 vaccination campaign was rolled out in 2014 yet data on vaccination coverage, prevalence of pneumococcal disease in children <5years are scarce. This study therefore evaluated the pneumococcal disease burden following pneumococcal conjugate vaccination campaign in Uganda.
Methods
This was an eight year retrospective analysis of pneumococcal surveillance data recorded from 3 regions of Uganda (namely Kigezi region in South Western Uganda, Busoga region in Eastern Uganda and Tooro region in Mid-western Uganda) between January 2012-December 2020. Demographic and clinical diagnosis data were abstracted from medical records at three regional referral hospitals representing the 3 regions to assess disease burden before and after introduction of the Pneumococcal conjugate vaccine (PVC 13) in Uganda. Data were summarized by demographic characteristics and geographical location of study subjects.
Results
Pre-vaccination period had a prevalence of 59.4% of the cases while the post-vaccination period had 40.6% prevalence rate. By region, prevalence was highest in Kigezi (52.1%) followed by Tooro (32.6%) and lowest in Busoga at (15.3%).
In these settings, immunological serotyping was never done and data on most risk factors were missing. The prevalence of paediatric pneumococcal infections remains high even after vaccination campaign calling for a review of the vaccination strategy.
Conclusion and recommendations
Pneumococcal disease burden was still high (40.6%) despite pneumococcal vaccination roll out. The highest prevalence of pneumococcal disease was in children under 24 months old. However, local surveillance capacity need to be strengthened to capture data on pneumococcal serotypes and pneumococcal disease risk factors.
Keywords
Burden; Pneumococcal Disease Burden, Pneumococcal Conjuate Vaccine; Uganda; Surveillance
Abbreviations
AEFI: Adverse events following immunization
DHIS2: District Health Information System
EHRs: Expanded Health Records
HMIS: Health Management Information System
IDSR: Integrated Disease Surveillance and Response
MOH: Ministry of Health
PCV: Pneumococcal Conjugate Vaccination
PHEOC: Public Health Emergency Operations Center
UNCST: Uganda National Council for Science and Technology
WHO: World Health Organisation
Introduction
Bacterial pneumonia is the leading preventable cause of childhood morbidity and mortality globally with many of the deaths occurring in low- and middle-income countries [1-2]. According to WHO, Streptococcus pneumonia (also known as Pneumococcus pneumonia) is the most common cause of pneumonia in children followed by Haemophilus influenzae typeb (Hib) globally [3]. Pneumococcal disease is a syndromic disease including severe Invasive Pneumococcal Disease (IPD) (e.g. pneumococcal meningitis, bacteremia) or less severe but frequent non-invasive pneumococcal disease (e.g. community-acquired pneumonia, bronchitis, otitis media, and sinusitis) [4]. About 90 distinct pneumococcal serotypes have been identified throughout the world, with a small number of these serotypes accounting for most disease in infants [5]. Pneumococcal vaccines are designed to cover the serotypes most frequently associated with severe pneumococcal disease [5].Two polysaccharide-protein conjugate vaccines have been on the market since 2009: the 10-valent (PCV10) and the 13-valent (PCV13) pneumococcal conjugate vaccines. Previously, a 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in early 2000, and at least 9 other pneumococcal conjugate vaccines containing 10–20 serotypes are undergoing trials in humans [5]. For instance, in the USA, Pneumococcal conjugate vaccines (PCV13, PCV15, and PCV20) and Pneumococcal polysaccharide vaccine (PPSV23) are recommended for all children younger than 5 years old [6]. The introduction of pneumococcal vaccines has led to a worldwide reduction in the incidence of pneumococcal infections and a related mortality in developed countries [4].
In 2007 WHO recommended to developing countries to include pneumococcal conjugate vaccines (PCV) in their infant routine immunization schedules [6]. The recommended PCVs for children included 10-valent pneumococcal conjugate vaccine (PCV10) and 13-valent pneumococcal conjugate vaccine (PCV13). Countries that introduced these vaccines have observed significant reductions in the incidence of severe (invasive) pneumococcal disease and community-acquired pneumonia [6]. By the end of 2020, 76.3% (148/194) of WHO member states had rolled out PCVs into their National Immunization Programmes [6]. Despite an overall decline in incidence of pneumococcal infections, pneumococcal diseases still remain a major public health problem worldwide, particularly in developing countries [4]. According to the World Health Organization (WHO) in 2015, of the global deaths among <5 years old children estimated at 5.83 million, 294,000 deaths were attributed to pneumococcal infections [4].
In Uganda, despite PCV roll out in 2014, pneumonia was the fourth leading cause of death in children under five years of age in 2017-18 [7]. A study that assessed the prevalence of pneumonia among children under 5years in in the post-vaccination period in western Uganda showed a prevalence of 25.6% [8]. Another study at National Referral Hospital in central Uganda reported a prevalence rate of 53.7% [9]. A systematic review and meta-analysis of data on pneumonia across East African countries, estimated the average prevalence of pneumonia in under-fives at 34% [10].
This study assessed the burden of pneumococcal disease among under 5 years old in the pre- and post-vaccination period (2012-2020) to gain more insight on vaccination coverage, pneumococcal burden over time and pneumococcal clinical characteristics in three regions of Uganda.
Materials and Methods
Study design and data collection
This was a retrospective, serial cross-sectional study in which medical records of children aged 5yrs and below for the period 1st January 2012 to 31st December 2020 were reviewed. The study utilized available local surveillance data from 3 regions of Uganda (namely Kigezi region in South western Uganda, Busoga region in Eastern Uganda and Tooro region in Mid-western Uganda). Demographic and clinical diagnosis data were abstracted from medical records at three regional referral hospitals representing the 3 regions to assess disease burden before and after introduction of the Pneumococcal conjugate vaccine (PVC 13) in Uganda
Statistical analysis
Data were collected using the Kobo-collect software and analysed using Stata 13. Demographic data were summarized using frequency Tables (1-6). Pneumococcal disease burden (prevalence) was calculated as a proportion of children clinically diagnosed with pneumococcal disease divided by all the children under risk of developing the disease.
|
Characteristics |
Frequency, n (%) |
|
Age (Months) Mean 0-24 25-48 >48 |
17.2 2,999 (82) 481(13.1) 179 (4.9) |
|
Sex Female Male |
1,709 (46.7) 1,953(53.3) |
|
Region Busoga Kigezi Tooro |
524(14.3) 2,012 (54.8) 1,137(30.9) |
Table1: Demographic characteristics (N=3662).
|
Condition |
Frequency, n(%) |
|
Pneumonia (n=3662) |
3428 (93.6%) |
|
Fever (n=3427) |
3261 ( 95.16%) |
|
Upper respiratory tract infection (mainly common cold) (n=3427) |
2780 (81.27%)
|
|
Sinusitis (n=3015) |
646 (21.43%) |
|
Ear infection/Otitis media (n=3008) |
75 (2.49% |
|
Brain/spinal cord infection/meningitis |
42 (1.4%) |
Table2: Pneumococcal disease clinical presentation.
|
Risk factor (n=3423) |
Freq. |
Percent |
|
HIV positive children |
51 |
1.49 |
|
Alcohol consumption by care giver |
25 |
0.73 |
|
Smoking by care giver |
27 |
0.79 |
|
COPD |
2 |
0.06 |
|
Asplenia |
1 |
0.03 |
|
Chronic renal disease |
8 |
0.23 |
|
Unknown |
3309 |
96.67 |
|
Total |
3423 |
100.00 |
Table 3: Pneumococcal disease risk factors.
|
Vaccination status |
Freq. |
Percent |
|
Not vaccinated/unknown |
2427 |
66.22 |
|
Vaccinated |
1238 |
33.78 |
|
Total |
3665 |
100.00 |
Table 4: Vaccination coverage.
|
Status |
Pneumococcal conjugate vaccine (PCV) n(%) |
Meningococcal vaccine n(%) |
Haemophilus influenzae typeb Vaccine (Hib) n(%) |
|
No/Data not available |
3,246 (88.58) |
3,252 (88.76) |
3,256 (88.87) |
|
Yes |
418(11.41) |
412(11.24) |
408(11.13) |
|
Total |
3,664 |
3,664 |
3,664 |
Table 5: Vaccination coverage by type of vaccine.
|
Serotype |
Frequency |
Percent |
Cum |
|
Data not available |
3,111 |
84.93 |
84.93 |
|
H. influenzae |
2 |
0.05 |
84.98 |
|
S. pneumoniae |
550 |
15.02 |
100.00 |
|
Total |
3,663 |
100 |
|
Table 6: Pneumococcal aetiological serotypes.
Ethical considérations
Ethical approvals was sought from Mulago Hospital Research and Ethics Committee (Approval Number MHREC 2165), Uganda National Council for Science and Technology (UNCST) (Research Registration Number with the UNCST : HS2102ES). The data collected was treated with utmost confidentiality and no participant’s identifiers were recorded during the data collection process. All relevant authorizations were obtained before proceeding with the study.
Results
Records for 3662 children 5years and below were retrieved, relevant data abstracted and analyzed. 46.7% were female, mean age was 17.2 months and majority (54.8%) were from Kigezi region in South-western Uganda
Overall period prevalence (2012-2020) was 93.43% with male children accounting for 53% of the disease burden
Majority of the cases were in the age category 0-24motnhs (82%) followed by age category 25-48 months and the least above 48months
The highest proportion of the cases (52.1%) were recorded from Kigezi region and the least from Busoga region (15.3%)
Most of the cases (42.3%) were recorded during the pre-vaccination period and the post-vaccination early/shortly after the introduction of the Pneumococcal Conjugate Vaccine (PCV) accounting for 40.6%. (Figures (1-4))
Diagnosis in all the study sites was mainly symptomatic/clinical. Aware that pneumococcal disease can be non-invasive (e.g. community-acquired pneumonia, otitis media, sinusitis) or invasive (e.g. meningitis, bacteremia), non-invasive pneumococcal disease was most common (93.6%) and invasive pneumococcal disease was only present in 1.4% (n=42/3008) of the study.
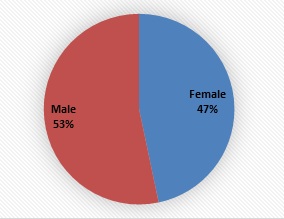 Figure 1: Pneumococcal disease burden by gender.
Figure 1: Pneumococcal disease burden by gender.
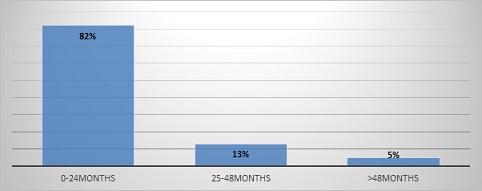 Figure 2: Pneumococcal disease burden by gender.
Figure 2: Pneumococcal disease burden by gender.
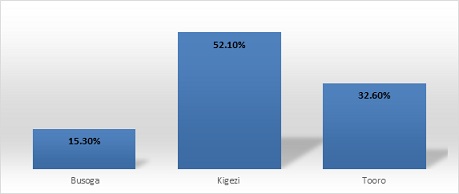 Figure 3: Pneumococcal disease burden by region.
Figure 3: Pneumococcal disease burden by region.
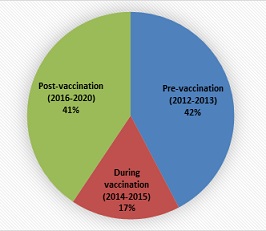 Figure 4: Pneumococcal disease burden by time period.
Figure 4: Pneumococcal disease burden by time period.
The known risk factors for pneumococcal disease are asplenia, alcoholism, smoking, diabetes mellitus, liver disease, lung disease, chronic heart disease, chronic kidney disease, chronic obstructive pulmonary disease (COPD) and immunocompromised conditions. However, data on most these risk factors were missing in all the study sites may be because they are rare among children and data on caregivers/parents were not captured.
The records retrieved indicated that for more than a half of the children’s vaccination status was unknown/not recorded (66.2 %, n=2,427) and only 34% (n=1238) were vaccinated using any of the 3 vaccines against pneumonia.
The recorded pneumococcal conjugate vaccine (PCV) coverage was at 11.4%, meningococcal vaccine coverage was 11.24% and 11.13 for the Haemophilus influenzae type b vaccine.
Aware that there are over 100 distinct pneumococcal serotypes and the conjugate pneumococcal vaccine usually target specific serotypes/groups , we however, found a lot of missing data on immunological serogroupings/serotypes meaning serotyping was never done in these settings. We only found some data on pneumococcal aetiological types (i.e. Haemophilus influenzae pneumonia, Streptococcus pnuemoniae pneumonia, etc). The most prevalent pneumococcal aetiological serotype was S. pneumoniae at 15% compared to H.influenzae at 0.05%.
Discussions
This study assessed the burden of pneumococcal disease among children below 5years and determined the distribution of the pneumococcal serotypes in under-5yrs old children including the risk factors before, during and after PCV13 rollout in Uganda. From the3662 records for children 5years and below that were retrieved, it was found out that 46.7% were female, mean age was 17.2 months, 0-24motnhs (82%) and majority (54.8%) were from Kigezi region in South-western Uganda. This could be due to weak immunity as the majority were young and at that age there is exposure to unclean environment as these children start to move by themselves. The findings are in agreement with those of a cross sectional study conducted at a Teaching Hospital in Bushenyi District, Western Uganda to determine the prevalence and associated factors of pneumonia among under-fives with acute respiratory symptoms inn which it was found out that age increased the risk of pneumonia by 3 times that is age below 6 months (OR=3.2, 95%CI=1.17–8.51, p=0.023) [11], other studies in Ethiopia and Malawi found out that children at age rang 2-12 months were 4 times more likely to develop pneumonia as compared to older age groups and children who were aged 2–12 months were 2.5 times more likely to develop pneumonia as compared to children above 12 months respectively [12,13].
The overall period prevalence (2012-2020) was 93.43% with male children accounting for 53% of the disease burden, the highest proportion of the cases (52.1%) were recorded from Kigezi region and the least from Busoga region (15.3%). This prevalence is low compared to findings in a study at Mulago National Referral Hospital Uganda which recorded prevalence of pneumonia in under-fives at 53.7% [14]. This could be due to the fact that the study was carried out in rural hospitals which serve children from rural areas that likely to be affected by environmental pollution, overcrowding and exposure to smoke due to indoor cooking with biomass which predispose to pneumonia comapred to Mulago Hospital which is in the city. The findings are inline with a systematic review and meta-analysis in East Africa, a study done in Ethiopia and Brazil which reported high odds of pnuemonia among children from rural areas compared to children from urban areas [15-17].
Regarding the period prevalence pre-vaccination and post-vaccination, most of the cases (42.3%) were recorded during the pre-vaccination period and the post-vaccination early/shortly after the introduction of the Pneumococcal Conjugate Vaccine (PCV) accounting for 40.6%. This reduction could be attributed to the introduction of the PVC into the routine immunisation schedule for the children as the unvaccinated children are likely to have weak immunity and increased probability of acquiring infections including pneumonia. This result was similar with studies conducted in Brazil, Bellary and India [18-20] and a systematic review conducted by Jackson et al, [21].
Only clinical diagnosis was used to classify pneumococcal disease as either non-invasive ( community-acquired pneumonia, otitis media, sinusitis) or invasive (e.g. meningitis, bacteremia), non-invasive pneumococcal disease was most common (93.6%) and invasive pneumococcal disease was only present in 1.4% (n=42/3008) of the study. This can attributed to the large numbers of patients that seen at these study sites and among other reasons understaffing and lack of confirmatory laboratories, difficulty to confirm microbiologically and imaging investigations yet it would be more appropriate to have the exact diagnosis if appropriate medication is to be given. This is backed by evidence of disappearing microbiology for documented by experts at Johns Hopkins Hospital [22, 23].
Limitations
Though our desire was to describe the clinical characteristics of childhood pneumonia by age, clinical presentation and risk factors over time but with secondary routine data, our analysis was limited to available data. Aware that the main risk factors for pneumococcal infections are asplenia, alcoholism, smoking, diabetes mellitus, liver disease, lung disease, chronic heart disease, chronic kidney disease, Chronic Obstructive Pulmonary Disease (COPD) and immunocompromised conditions, we found out that data on most risk factors were missing may be because they are rare among children and caregivers’/parents’ data were not captured.
Aware that there are over 100 distinct pneumococcal serotypes and the conjugate pneumococcal vaccine usually target specific serotypes/groups , we however, found a lot of missing data on immunological serogroupings/serotypes meaning serotyping was never done in these settings. We only found some data on pneumococcal aetiological serotypes/groups (i.e. Haemophilus influenzae pneumonia, Streptococcus pnuemoniae pneumonia)
Conclusion and Recommendations
Pneumococcal disease burden was still high (40.6%) despite pneumococcal vaccination roll out. The highest prevalence of pneumococcal disease was in children under 24 months old. One of the limitation of this study was missing data and poor record keeping since most of the patient information was manually kept on file and difficult to retrieve. There is therefore need for adoption of computerized data storage systems for safety and ease of access of local surveillance data. Additionally, local surveillance capacity need to be strengthened to capture data on pneumococcal serotypes and pneumococcal disease risk factors.
Acknowledgments
Collate acknowledgments in a separate section at the end of the article before the references. List here those individuals who provided help during the research.
Conflicts of interest
The study team declares no conflict of interest
References
- WHO (2015) Causes of child mortality, 2000-2012. Geneva: World Health Organization.
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 374: 893-902.
- https://www.who.int/news-room/fact-sheets/detail/pneumonia
- https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/pneumococcus
- World Health Organization (2019) Weekly epidemiological record 94: 85-104.
- https://www.cdc.gov/vaccines/vpd/pneumo/index.html
- Ministry of Health, Uganda (2018) Annual Health Sector Performance Report 2017/2018. Ministry of Health: Kampala, Uganda
- Kiconco G, Turyasiima M, Ndamira A, Yamile OA, Egesa WI, et al. (2021) Prevalence and associated factors of pneumonia among under-fives with acute respiratory symptoms: A cross sectional study at a Teaching Hospital in Bushenyi District, Western Uganda. Afr Health Sci 21: 1701-1710.
- Nantanda R, Tumwine JK, Ndeezi GOM (2013) Asthma and Pneumonia among Children Less Than Five Years with Acute Respiratory Symptoms in Mulago Hospital, Uganda: Evidence of Under-Diagnosis of Asthma. PLoS One 8: e81562.
- Biruk B, Melaku B, Ayelign M, Mesfin W, Molla A, et al. (2020) Prevalenc of pneumonia and its associated factors among under-five children in East Africa: A systematic review and meta-analysis. BMC Pediatr 20: 254.
- Kiconco G, Turyasiima M, Ndamira A, Yamile OA, Egesa WI, et al. (2021) Prevalence and associated factors of pneumonia among under-fives with acute respiratory symptoms: A cross sectional study at a Teaching Hospital in Bushenyi District, Western Uganda. Afr Health Sci 21:1701-1710.
- Abuka T (2017) Prevalence of pneumonia and factors associated among children 2-59 months old in Wondo Genet district, Sidama zone, SNNPR, Ethiopia. Curr Pediatr Res 21: 19-25.
- Uwemedimo O, Lewis T, Essien E, Grace JC, Humphreys N, et al. (2018) Distribution and Determinants of Pneumonia Diagnosis Using Integrated Management of Childhood Illness Guidelines: A Nationally Representative Study in Malawi. BMJ Glob Health 3: E000506.
- Nantanda R, Tumwine JK, Ndeezi G, Ostergaard MS (2013) Asthma and Pneumonia among Children Less Than Five Years with Acute Respiratory Symptoms in Mulago Hospital, Uganda: Evidence of Under-Diagnosis of Asthma. PLoS One 8: e81562.
- Biruk B, Melaku B, Ayelign M, Mesfin W, Molla A, et al. (2020) Prevalenc of pneumonia and its associated factors among under-five children in East Africa: A systematic review and meta-analysis. BMC Pediatr 20: 254.
- Fonseca Lima EJ, Mello MJG, Albuquerque M de FPM de, Lopes MIL, Serra GHC, et al. (2016) Risk factors for community-acquired pneumonia in children under five years of age in the post pneumococcal conjugate vaccine era in Brazil: A case control study. BMC Pediatrics 16: 1-9.
- Da Fonseca Lima EJ, Mello MJG, Lopes MIL, Serra GHC, Lima DEP, et al. (2016) Risk factors for community-acquired pneumonia in children under five years of age in the post-pneumococcal conjugate vaccine era in Brazil: A case control study. BMC Pediatr 16: 157.
- Hemagiri K, Sameena A, Aravind K, Khan W, Vasanta S (2014) Risk factors for severe pneumonia in under five children-a hospital based study. Int J Res Health Sci 2: 47-57.
- Gothankar J, Doke P, Dhumale G, Pore P, Lalwani S, et al. (2018) Reported incidence and risk factors of childhood pneumonia in India: A community-based cross-sectional study. BMC Public Health 18: 1-11.
- Jackson S, Mathews KH, Pulani? D, Falconer R, Rudan I, et al. (2013) Risk factors for severe acute lower respiratory infections in children–a systematic review and meta-analysis. Croatian Med J 54: 110-121.
- Fekety FRJr, Caldwell J, Gump D, Johnson JE, Maxson W, et al. (1971) Bacteria, viruses, and mycoplasmas in acute pneumonia in adults. Am Rev Respir Dis 104: 499-507.
- Dans PE, Charache PC, Fahey M, Otter SE (1984) Management of pneumonia in the prospective payment era: A need for more clinician and support service interaction. Arch Intern Med 144: 1392-1397.
- Mundy LM, Auwaerter PG, Oldach D, Warner ML, Burton A, et al. (1995) Community-acquired pneumonia: Impact of immune status. Am J Respir Crit Care Med 152: 1309-1315.
Citation: Antony I, Rubaihayo J, Atuhe I, Mujawimana A, Ndungutse D (2022) Burden of Pneumococcal Disease: An 8-Year Retrospective Analysis of Local Surveillance Data from Three Regions in Uganda (2012-2020). J Vaccines Res Vaccine 8: 21.
Copyright: © 2022 Ikiriza Antony, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

