
Chronic Fatigue Syndrome: An Autoimmune Disorder of the Neuroendocrine System
*Corresponding Author(s):
Khoa D NguyenDepartment Of Pathology, Stanford University School Of Medicine, California, United States
Email:kdnguyen@stanford.edu
Abstract
Chronic Fatigue Syndrome (CFS) is a complex multi-system disorder with unknown etiology. While several abnormalities of the neuroendocrine and immune systems have been independently implicated in CFS, the involvement of aberrant neuro-immune cross-talks in the pathogenesis of this disorder has not been well explored. Here we put forward a medical hypothesis that would invite further investigation into the potential autoimmune nature of CFS. Clinical evidences will be outlined to support the idea thatautoimmune attacks against cortisol, a major neuroendocrine hormone of the Hypothalamic-Pituitary-Adrenal (HPA) axis, might be the underlying cause of fatigue, the cardinal pathology of CFS.
Keywords
Autoimmunity; Chronic Fatique Syndrome; Cortisol; Hypothalamic-pituitary-adrenal axis
ABBREVIATIONS
ACTH: Adrenocorticotropic Hormone; CFS: Chronic fatigue syndrome; CRH: Corticotropin-Releasing Hormone; CORT: Cortisol; HPA: Hypothalamic-Pituitary-Adrenal Axis; MS: Multiple Sclerosis; PEM: Post Exertional Malaise
INTRODUCTION
Chronic Fatigue Syndrome (CSF), also referred to as myalgic encephalomyelitis or systemic exertion intolerance disease, is characterized by extreme fatigue or tiredness that doesn’t go away with rest and can’t be explained by an underlying medical condition. CFS is a gender-bias disorder, in which females are more likely to develop the illness [1]. Furthermore, those with family members who are diagnosed with CSF are more likely to develop this condition, suggesting the presence of a genetic predisposition to CFS [2]. This enigmatic disorder consists of a wide range of symptoms, such as memory loss, muscle or joint pain, fatigue and prolonged exhaustion after physical or mental exercise (post-exertional malaise) [3]. Because of the pathological heterogeneity of CFS patients, studying hallmark symptoms of this disorder such as fatigue and post-exertional malaise are critical for further understanding of its pathological mechanisms. Furthermore, understanding and identifying similarities between CFS and other medical illnesses could also be useful for delineating the primary cause(s) of this disorder.
Fatigue as the pathological hallmark of CFS
Fatigue is one of the most common CFS symptoms, which has a major impact on life quality of CFS patients [3,4]. Fatigue can be defined as the lack of energy to complete voluntary physical and mental functions. Fatigue can occur as a result of drastic fluctuations in normal bodily functioning or as a symptom of other medical conditions, such as heart disease, depression and autoimmunity [5-7]. However, fatigue in autoimmunity is likely to result from a different underlying pathology than fatigue in heart disease or other neurological conditions. Abnormal immune functions might result in fatigue in autoimmunity while fatigue associated with other neurological conditions might be the result of a multitude of factors such as sleep pattern disruption, depression, or neuroendocrine changes [6]. For example, fatigue is a common symptom of several autoimmune diseases: 98% of patients with autoimmune diseases suffer from fatigue and this symptom was reported to be the primary cause of autoimmunity-related depression and behavioral incompetency [8]. Fatigue is also a prominent feature of Multiple Sclerosis (MS), a neurodegenerative disease with female gender-biased like CFS [9,10]. 70-90% of MS patients suffer from fatigue, which is often the most disabling symptom in this disease [9]. While the precise pathological mechanism of fatigue in neurological diseases remains unknown, disturbance of neurotransmitter signaling pathways as a consequence of primary neuronal defects in this disease might be the underlying cause of this symptom.
CFS as a multi-system disorder
Accumulating evidences have suggested that abnormalities in the neuroendocrine and immune systems are involved in the manifestation of CFS-related fatigue. In this regard, fatigue is often associated with some neurometabolic conditions as well as immune dysregulation during autoimmunity. These findings will be discussed in details below to provide a novel perspective on how immunity and neuroendocrine system could be intimately involved in the modulation of fatigue in CFS.
Involvement of the neuroendocrine system in CFS
Post-exertional Malaise (PEM), a unique type of fatigue that is aggravated by physical and mental stresses in CFS, has been showed to be associated with unique changes in gene expression ofneurological and metabolic processes [11-13]. In this study, exercise induced genes encoding some adrenergic receptors in a major subgroup of CFS patients while others exhibit a marked reduction in other receptors belong to this family upon exercise, suggesting the involvement of the nervous system in CFS [12]. Interestingly, disturbances in the Hypothalamic-Pituitary-Adrenal (HPA) axis have also been documented CFS. This signaling pathway is activated in CFS patients in response to stress stimuli, rangingfrom physical, mental, and emotional stress to sensitivity to noise, light and heat. Stressors with greater magnitude will result in more severe fatigue in CFS. Notably, circulating cortisol, a major stress hormone that is released upon HPA activation, is reduced in some CFS patients in compared to healthy controls [14]. The natural “stress” of awakening also induced a blunted cortisol release in CFS patients [15]. Furthermore, CFS severity is negatively correlated with cortisol level. Conversely, CFS recovery is associated withnormalization of this stress hormone, suggesting a potential role of this molecule in the development and resolution of CFS-related fatigue [16]. These genetic and post-transcriptional changes of the neuroendocrine system might represent the physiological maladaptation of the body to stressors, resulting in fatigue-like symptoms. Lastly, a subset of CFS patients notably undergoes remission during pregnancy [17]. This fatigue relief has been linked to the pregnancy-associated alterations of hormones such as lactogen, relaxin, and progesterone [18,19]. Together, these observations provide convincing evidences that neuro-metabolic changes may be involved in the development of fatigue in CFS.
The involvement of the immune system in CFS
Immune system has been long suggested to be involved in the pathogenesis of CFS. When the immune system is suppressed, such as during pregnancy to ensure that the maternal immunological responses will not recognize the fetus as “foreign”, some CFS patients experience fatigue relief [17,18]. Another example in which suppression of immunity results in fatigue relief is in the context of rituximab- induced CFS remission [20]. Rituximab is a monoclonal antibody that binds to the CD20 protein on the surface of B cells, causing their depletion by a variety of mechanisms, and therefore is used to successfully treat B-cell mediated autoimmune conditions, such as MS [21]. In this study, sustainable reduction in the symptom of fatigue was reported in 67% of CFS patients who received rituximab in comparison to 13% of those in the placebo group after a 12-month follow-up [22,23]. However, a recent study showed that rituximab treatment was not associated with alleviation of CFS symptoms [24], suggesting that B cells might represent a pathological mechanism at play only in a subset of CFS subjects. Nevertheless, these observations are consistent with the hypothesis that fatigue, the pathological hallmark of CFS, might be associated with abnormal activation of the immune system.
Is CFS an autoimmune disorder of the neuroendocrine system
Several similarities between MS and CFS such as the induction of symptom relief by the same pharmacologic agent as well as during pregnancy, and the (female) gender bias of both conditions suggest that CFS might represent an autoimmune disorder with a unique involvement of the neuroendocrine system. In this regard, the reduced level of cortisol in some CFS patients point towards a hypothesis in which increased autogenic stimulation of the immune system against this stress hormone of the HPA axis might be involved in the modulation of CFS-relatedfatigue.
HPA Signaling Axis
The HPA axis is responsible for the release the neuroendocrine mediators of the fight or flight response. This response is characterized by hypothalamic release of Corticotropin-Releasing Hormone (CRH). When CRH binds to its receptor on the anterior pituitary gland, Adrenocorticotropic Hormone (ACTH) is released. ACTH subsequently engages with its receptor on the adrenal cortex, stimulating cortisol release. In response to stressors, cortisol will be released for several hours after encountering the stressor. After reaching a threshold level in the circulation, cortisol exerts negative feedback to the hypothalamic release of CRH and the pituitary release of ACTH, returning the system to homeostasis. Thus, there is strong interdependency of the release of these three major HPA hormones in mounting appropriate response to stressors with varying intensities (Figure 1).
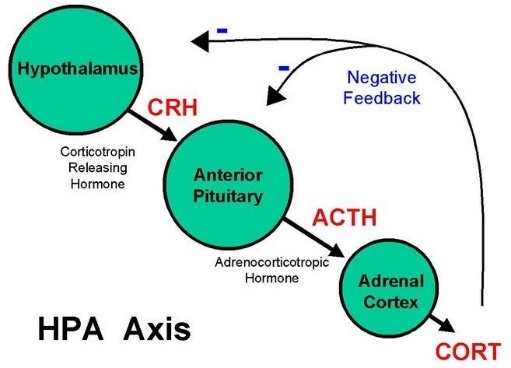 Figure 1: Molecular Components of HPA axis
Figure 1: Molecular Components of HPA axis
HPA hormones as autoantigens in CFS
In classical autoimmune conditions such as Rheumatoid Arthritis (RA) or MS, damage due to aberrant targeting of self-antigens would accumulate and is easily discerned. For example, in RA, whenever the immune system is targeting the synovial joints, symptoms such as joint pain, joint swelling, joint stiffness and loss of joint mobility occurs [25,26]. In MS, whenever the immune system is attacking nervous system, patients experience difficulties with vision, movement, and speech. In contrast, the damages due to autoimmune attack against hormones would not accumulate significantly over time due to the short half-lives of these molecules. Therefore, while this would be consistent with the notion that CFS is an “invisible” illness, proving the presence of an autoimmune attack against a naturally short-lived auto-antigen would not betrivial .Furthermore, even if autogenic hormone exists, correlating the severity of the autoimmune reaction with clinical outcome, fatigue in this case, would also be challenging because subjective metrics to quantify fatigue have not been well defined. Nevertheless, there are several clinical studies outlining the evidence of HPA hormones as autogenic targets in CFS, suggesting that dysregulation of the HPA signaling axis might be the chief underlying cause of CFS.
Dysregulation of cortisol production has also been documented as a unique feature of CFS as well as a potentially novel biomarker for PEM [14-16]. Besides these abnormalities, in ACTH stimulation assay where actual stressors are mimicked in order to trigger cortisol release, CFS patients produced significantly less cortisol (Table 1). Furthermore, some studies showed the kinetics of cortisol production in ACTH stimulation revealed an initial exaggeration in cortisol level, followed by a drastic reduction in this hormone [16]. This observation implies that rapid removal of cortisol is accountable for the abnormal cortisol kinetics in CFS patients, raising the possibility that cortisol might be the autogenic target in CFS. If this hypothesis on the autoimmune nature of CFS-related fatigue is correct, HPA stimulation would trigger a greater production of hormonal antigens. These molecules are subsequently marked for destruction by the immune system, causing an increase in symptom of fatigue. On the other hand, CFS- related fatigue should be able to be reduced by restricting the activation of HPA axis, which might have been the underlying cause for the success of graded exercise and pacing therapies for CFS patients [27]. This rationale would be the foundation for an empirical scientific test to evaluate the neuroendocrine/autoimmune hypothesis of CFS, hopefully in the near future. It’s worth noting that while cortisol level was clearly diminished in these studies (Table 1), hypocortisolism was only present in a very small subset of CFS patients, reflecting the complex heterogeinity of this disease as well as the potential need to use cortisol as a bio-marker for further disease sub-classification in mechanistic studies of CFS.
|
Study |
Subjects |
Illness duration (years) |
Method |
Cortisol findings in CFS |
|
Demitrack et al. [27] |
19 CFS vs. 17 healthy controls |
7.2 |
3 samples at 20:00:00 |
Low level |
|
Moorkenset al. [28] |
73 CFS vs. 21 healthy controls |
1.5 |
5 samples between 22:00 and 06:00 |
Low level |
|
Hamilos et al. [29] |
7 CFS vs. 7 healthy controls/ 14 disease controls |
Not Available |
7 samples over 24 hour period |
Lower peak level |
|
MacHaleet al. [30] |
30 CFS vs. 15 healthy controls |
5.2 |
2 samples at 08:00 and 22:00 |
Reduced diurnal variations |
Table 1: Cortisol levels in CFS subjects in ACTH stimulation assays.
SUMMARY
We highlighted the major findings in clinical and experimental research that point towards a potential explanation for the multifactorial nature of CFS, in which abnormalities at the crossroad of immunity and neuroendocrine system might be the underlying cause of this illness. The hypothesis that CFS is an autoimmune disease is a strong fit for the pathological manifestation of a subtype of this disease, given the similarities between CFS and other autoimmune-mediated conditions as well as the positive response of some CFS patients to medications proven to be efficacious for autoimmune diseases. Furthermore, the striking correlations between dysfunctions of HPA axis and fatigue symptoms allows us to postulate that molecular mediators of this signaling pathway, such as the stress hormone cortisol, might be the primary auto-antigenic targets for immune-mediated removal. While this novel medical hypothesis provide plausible explanations for major pathological features of CFS, empirical validations in clinical studies of CFS subjects (synthetic ACTH stimulation, autoantibody identification, stress hormone biomarker discovery) as well as in experimental models (mechanisms of CFS remission induced by pregnancy or rituximab) are needed to confirm the potential involvement of aberrant neuro-immune interaction in this illness.
ACKNOWLEDGEMENT
R.A.W developed the original hypothesis and wrote the manuscript with critical revision from K.D.N.
CONFLICT OF INTEREST
K.D.N is the scientific founder of Tranquis Therapeutics and received consulting payment from Tranquis Therapeutics. R.A.W declared no conflict of interests.
REFERENCES
- Lewis I, Pairman J, Spickett G, Newton JL (2013) Is chronic fatigue syndrome in older patients a different disease? -- a clinical cohort study. Eur J Clin Invest 43: 302-308.
- Albright F, Light K, Light A, Bateman L, Cannon-Albright LA (2011) Evidence for a heritable predisposition to Chronic Fatigue Syndrome. BMC Neurol 11: 62.
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine (2015) Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington (DC): National Academies Press (US).
- Roberts D (2018) Chronic Fatigue Syndrome and quality of life. Patient Relat Outcome Meas 9: 253-262.
- Nealson R, Dar Y, Thomas K, Dimsdale J (2008) The Relationship between Fatigue and Cardiac Functioning. Arch Intern Med 168: 943-949.
- Targum SD, Fava M (2011) Fatigue as a Residual Symptom of Depression. Innov Clin Neurosci 8: 40-43.
- Morris G, Berk M, Walder K, Maes M (2015) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13: 28.
- American Autoimmune Related Diseases Association (AARDA) (2015) Profound, debilitating fatigue found to be a major issue for autoimmune disease patients in new national survey. Science Daily.
- Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ (1994) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21: 9-14.
- Harbo HF, Gold R, Tintoré M (2013) Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord 6: 237-248.
- Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, et al. (2012) Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med 271: 64-81.
- Light AR, White AT, Hughen RW, Light KC (2009) Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain 10: 1099-1112.
- Keech A, Vollmer-Conna U, Barry BK, Lloyd AR (2016) Gene Expression in Response to Exercise in Patients with Chronic Fatigue Syndrome: A Pilot Study. Front Physiol 7: 421.
- Scott LV, Medbak S, Dinan TG (1998) Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatr Scand 97: 450-457.
- Hall DL, Lattie EG, Antoni MH, Fletcher MA, Czaja S et al. (2014) Stress management skills, cortisol awakening response, and post-exertional malaise in Chronic Fatigue Syndrome. Psychoneuroendocrinology 49: 26-31.
- Nijhof SL, Rutten JM, Uiterwaal CS, Bleijenberg G, Kimpen JL, et al. (2014) The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology 42: 199-206.
- Underhill R (2009) Pregnancy in Women with Chronic Fatigue Syndrome (ME/CFS) 2009. NJCFSA pg. no: 1-2.
- Allen PR (2008) Chronic Fatigue Syndrome: Implications for Women and Their Health Care Providers during the Childbearing Years. J Midwifery Womens Health 53: 289-301.
- Fluge Ø, Risa K, Lunde S, Alme K, Rekeland IG, et al. (2015) B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS One 10: 7.
- Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, et al. (2016) Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy. Neurology 87: 2074-2081.
- Fluge Ø, Mella O (2009) Clinical impact of B-cell depletion with the anti-CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series. BMC Neurol 9: 28.
- Fluge Ø, Bruland O, Risa K, Storstein A, Kristoffersen EK, et al. (2011) Benefit from B- lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS One 6: e26358.
- Fluge Ø, Rekeland IG, Lien K, Thürmer H, Borchgrevink PC, et al. (2019) B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Intern Med 170: 585-593.
- Ostensen M, Villiger PM (2007) The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol 29: 185-191.
- Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, et al. (2018) Rheumatoid Arthritis: A Brief Overview of the Treatment. Med Princ Pract 27: 501-507.
- Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM (2004) Randomised controlled trial of graded exercise in chronic fatigue syndrome. Med J Aust 180: 444-448.
- Demitrack MA, Gold PW, Dale JK, Krahn DD, Kling MA, et al. (1992) Plasma and cerebrospinal fluid monoamine metabolism in patients with chronic fatigue syndrome: preliminary findings. Biol Psychiatry 32: 1065-1077.
- Moorkens G, Berwaerts J, Wynants H, Abs R (2000) Characterization of pituitary function with emphasis on GH secretion in the chronic fatigue syndrome. Clin Endocrinol (Oxf) 53: 99-106.
- Hamilos DL, Nutter D, Gershtenson J, Redmond DP, Clementi JD, et al. (1998) Core body temperature is normal in chronic fatigue syndrome. Biol Psychiatry 43: 293-302.
- MacHale SM, Cavanagh JT, Bennie J, Carroll S, Goodwin GM, et al. (1998) Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology 38: 213-217.
Citation: Nguyen KD, Wills RA (2019) Chronic Fatigue Syndrome: An Autoimmune Disorder of the Neuroendocrine System. J Biotech Res Biochem 2: 004.
Copyright: © 2019 Khoa D Nguyen, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

