
Clinical Approach of Novel Autologous Stem Cell Therapy for Primary Ovarian Insufficiency and Endometrial Regeneration
*Corresponding Author(s):
Vaman GhodakeIVF Lab, Dr. Ghodake Advanced Laparoscopy Surgery And Test Tube Baby Centre, Sangli, Maharshtra-416416, India
Email:drvamanghodake@gmail.com
Abstract
Bone Marrow-Derived Mesenchymal Stem Cell Fraction (BMSCF) therapy has various applications as potential use in regenerative medicine. Here, we present an original case of Primary ovarian insufficiency associated with poor ovarian reserves and thin endometrium in a woman who achieved a live birth with BMSCF supplemented with PRP (platelet rich plasma). The age between 24 to 42-year-old woman (n=10) came to our centre with primary infertility. Her husband's sperm count and quality was normal. The patient was advised for fertility enhancing surgery and advised stem cell therapy for poor ovarian reserve and thin endometrium. Several attempts of IVF with Hormone Replacement Therapy (HRT) were made, but the desired thickness of the endometrium was not achieved. Intra ovarian and sub endometrial injection of BMSCF was given, followed by HRT for three months, which resulted in improved endometrium and ovary function. The treatment resulted in the conception and delivery of a 2.9 kg baby through lower segment caesarean section. Recently, there has been substantial interest in stem cells as a novel regenerative therapy for the regeneration of human endometrium disorders and poor ovarian reserves.
Introduction
Researchers have characterized stem cells and evaluated their therapeutic effects in various animal models of degenerative diseases [1]. Based on the considerable data on the feasibility of stem cells for various degenerative diseases, clinical trials are ongoing to develop new stem cell-based treatment strategies in regenerative medicine. However, there are few reports on the effects of stem cells in reproductive diseases [2]. In reproductive medicine, ovarian function is important in the health of both young and old women. Stem cell therapy has become a promising, particularly for indications not possible to treat medically or surgically to improve the condition. Stem cells have the ability to self-renew and differentiate into specific lineage [3]. MSCs can be isolated from various sources, including bone marrow, placenta, dental pulp [4-6]. The advantages of autologous MSCs are that they do not raise any ethical concerns; they have no immunogenicity and immunomodulatory function. Premature Ovarian Insufficiency (POI) is a clinical syndrome defined by loss of ovarian activity before the age of 40 years. POI is characterized by menstrual disturbance with raised gonadotropins, low Anti-Mullerian Hormone (AMH), and estradiol level [7]. Autologous BMSCF s was tried in POI to increase the follicular recruitment and avoiding the need for oocyte donation program and regeneration of endometrium. This study analyses the role of autologous BMSCF in POI and thin endometrium. In the absence of stem cell therapy, accepting oocyte donor program or adoption would have been the only viable options for this patient for which she was not ready. In particular, POI defines a state of female hypogonadism which causes a loss of ovarian function before the age of 40 years. POI can be caused by side effects of anticancer chemotherapy or by surgical means [8]. This condition can be diagnosed based on a decrease in the number of follicles, abnormalities in the menstrual cycle, and infertility. There are several preclinical studies of stem cell therapy to treat ovarian dysfunction in several animal models and endometrium regeneration [9,10].
Methodology
Ten patients, who had a history of cancelled cycles due to inadequate endometrial growth (less than 7 mm) in the past FET cycles despite standard treatments and POI, were recruited into the study performed in the IVF centre, in 2021. After approval from the Institutional Ethics Committee, 10 patients was selected after written informed consent after explaining the pros and cons of this procedure were included in the study. This prospective interventional study was designed for intraovarian instillation and endometrium infusion of BMSCFs along with PRP in 10 patients who are poor responders for fertility.
Inclusion criteria were as follows:
Age group of 20-45 years
- Poor responder (the expected poor responder group where AFC
- Normal karyotype
- Normal semen parameters
Exclusion criteria were as follows:
- Abnormal karyotyping
- Autoimmune diseases
- POD due to chemotherapy or radiotherapy
- Active viral infections
The AFC by transvaginal ultrasound and serum AMH levels were considered markers of ovarian response. Both were recorded in all patients under basal conditions before and 5 weeks after the procedure.
Bone marrow aspiration
Age between 24-45-year-old poor ovarian failures, poor ovarian reserve, thin endometrium female, came to our fertility clinic. Their AMH level was low 0.4 ng/mL and AFC ≤ 2. On ultrasonography, ovaries were unremarkable with antral follicle count of one. It was thought to use autologous BMSCF for the rejuvenation of functioning ovarian tissue and optimizing the success rate of achieving pregnancy through assisted reproduction. Patients with POD, undergo diagnosis and screening confirming diagnosis including history and physical exams, labs and diagnostic procedures. Following final approval and under anaesthesia, bone marrow aspiration with separation of the Bone Marrow Derived Stem Cell Fraction (BMSCF) will be performed. BMSCF harvested from the anterior superior iliac crest region. Bone marrow aspiration procedure (BMAP) was carried out by local anaesthesia with light sedation monitored by an anesthesiologist for access to the posterior superior iliac crest.
Preparation of Autologous Bone marrow-derived mesenchymal stem cell fraction
Bone marrow aspiration was done from the posterior superior iliac spine under local anaesthesia using the Jamshidi needle (13G) and 20-ml syringe prewashed with heparin maintaining strict asepsis. Prewashed the syringe avoids clot and coagulation, which can diminish the ultimate yield from the aspiration. Approximately 80-100 mL of bone marrow is aspirated. BMSCF were isolated by using automated closed-circuit centrifugation unit .By this method, we got 20 mL of stem cell concentrate. The stem cell mixed with PRP for the entire patient for transfusion. BMSCF were counted using a Neubauer chamber and cell viability was determined by counting the blue (dead) and transparent (alive) cells by trypan blue (Sigma) staining.
BMSCF infusion
Considering the small size of ovaries, we preferred laparoscopic instillation of BMSCF in ovaries. Ovaries were held at the cranial and caudal position with forceps and intra-ovarian instillation of about 1–2 ml of BMSCF at 3-4 sites performed bilaterally and sub endometrial 1-2 ml stem cells injected for endometrial regeneration (Figures 1&2).
 Figure 1: Ovary before BMSCF injection. Ovary after BMSCF injection.
Figure 1: Ovary before BMSCF injection. Ovary after BMSCF injection.
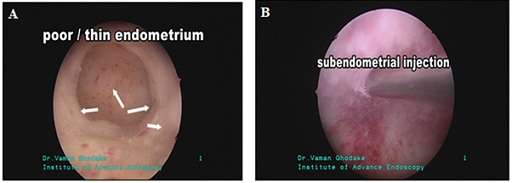 Figure 2: A) Thin endometrium, B) Sub-endometrial PRP+BMSCF Injection.
Figure 2: A) Thin endometrium, B) Sub-endometrial PRP+BMSCF Injection.
Results And Discussion
The bone marrow is promising sources of MSCs, and although its aspiration is invasive technique for the MSC isolation, it is the most evaluated approach for cell therapy. MSC differentiation potential and the viability of the bone marrow MSCs decrease with age. PRP is defined as a plasma fraction of autologous blood. It has been shown to improve tissue regeneration with the expression of cytokines and growth factors [11]. A few studies have evaluated the role of intrauterine instillation of PRP in endometrium regeneration [12-14]. It has been shown to improve embryo transfer and vascularity through releasing cytokines and growth factors, containing Vascular Endothelial Growth Factor (VEGF), transforming growth factor, platelet-derived growth factor, and epidermal growth factor [15]. These factors regulate cell proliferation and differentiation, and promote extracellular matrix accumulation.
After BMSCs transplantation in 45-year-old female to gives successful birth to a healthy baby. There are reports of pregnancy after intraovarian stem cell instillation, the mean duration for which their effect remains is still not fully understood. In our cases, the patient underwent cycle of IVF after stem cell therapy. The sonographic findings were suggestive of better ovarian volume, blood flow and antral follicle count. The patients were counselled to keep on trying naturally with the hope that the effect of stem cell therapy will remain in the subsequent cycles. The patients were followed up for 6-8 months with ultrasonography and conceived spontaneously in the subsequent years. But this therapy comes with some limitations such as genetic and karyotype abnormalities. The present report described 10 IVF cycles in patients who underwent BMSCF therapy with PRP. For successful implantation, the endometrium needs to regenerate and proliferate properly. In this complex process, different type of cell such as endothelial, epithelial, and stem cells and fibroblasts are involved. Growth factors, pro-inflammatory cytokines, and local chemokines also have important role in tissue regeneration. The bone marrow-derived mesenchymal stem cells (BMSCs) could restore the structure and function of injured tissues [16]. Recent studies suggest that stem cell therapy holds promise in treatment of variety of diseases including reproductive dysfunction. In a recent study, BMSC treated animals resumed ovarian function. In another investigation, granulosa cell apoptosis induced by cisplatin was reduced when BMSCs were migrated to granulosa cells in vitro [9]. In this study, chemotherapy-induced POI rats were injected with BMSCs. The BMSCs treatment group's antral follicle count and estradiol levels increased after 30 days, compared with untreated POI group. In a recent clinical trial evaluated the therapeutic potential of autologous mesenchymal bone marrow stem cells transplantation in women with POI [17]. Patients with POI were selected and their ovaries were injected with autologous BMSC at time of laparoscopy. The results shown continuation of menstruation in after 3 months; showed focal secretory changes after having atrophic endometrium. According to these results, BMSC seem to have the ability to resuscitate prematurely failed ovaries both in their hormonal and follicular development abilities.
Researchers recently reported that stem cell transplantation improves ovarian function which suggests a therapeutic potential [18,19]. The therapeutic effects of stem cells can be measured by several factors, such as folliculogenesis, the Granulosa Cells (GC) apoptosis rate, vascular formation, the pregnancy rate and the regulation of hormone levels. Folliculogenesis can be endorsed by the stem cells transplantation, which can trigger oogenesis. ADSCs are known to augment the severity of theca cells and GCs, resulting in an ideal environment for folliculogenesis [20,21]. PBMC contain various multipotent progenitor cells and have the ability to restore organ function and regenerate tissues. The combination of Platelet-Rich Plasma (PRP) and PBMCs has a synergistic effect on restoring folliculogenesis when transplanted into the induced Premature Ovarian Insufficiency (POI) model [21]. Our study shown that synergetic effect of autologous BMSCF and PRP therapy in a 24-year-old blighted ovum poor ovarian reserve helped in procuring a pregnancy and delivery of a healthy 2.9 kg baby through assisted reproduction. It also help to improve endometrium thicken for embryo implantation for IVF treatment (Table 1).
|
No |
Pt / age / married |
Diagnosis |
USG |
USG RT OVARY |
USG LT OVARY |
LAPAROSCOPY |
HYSTEROSCOPY |
Clinical |
Stem cell |
result |
|
1 |
KMC/27 /4 |
Twice aborted – blighted ovum Poor ovarian reserve |
Uterus normal
|
19x13x16 mm= VOLUME 2.3 ml AFC = 0-2 |
13x19x20 mm= VOLUME 2.9 ml AFC 0-2 |
uterus normal ovaries smaller atrophic |
Endometrial cavity normal |
Non responding well ovulation induction drugs Poor follicular response Endometrium thin not growing more than 6 mm |
Intra ovarian Subendometrial
opu - -4 eoocystes
3 embryos |
IVF 2950 gms Baby
|
|
2 |
VRM 24/ 2 |
Premature ovarian failure AMH = below 0.1 Fsh =70 Lh 24.56 Secondary amenorrhoea Responding well to medications |
Atrophic smaller 35x25x17 mm Thin central endometrial echo |
Smaller Could not be accessed properly |
Smaller Could not be accessed properly |
streak like smaller atrophic ovaries |
cavity smaller twice metroplasty done |
Ovarian failure Secondary amenorrhea Hormones correlating with post-menopausal age group Endometrium thin 2-3 mm Only spotting after withdrawal bleeding after medication |
Sub-endometrial stem cells
|
Oocyte donation Husband sperms Ivf done 1stivf aborted at 6 wks 2ndivf – Maximum endometrial thickmess – 6.5 mm Presently 14 wks viable pregnancy |
|
3 |
STJ 32 / 17 |
Poor responder |
Uterus normal |
20x25x29 mm Vol = 5.8 ml |
25x17x21 mm Vol = 5.2 ml |
Uterus normal Ovaries appears smaller |
Endometrial cavity normal Poor endometrial quality |
Endometrium is poor and took almost 2 yrs for endometrial preparation Finally endometrium was 7.4 mm thick with zone 5 vascularity IVF – FET done |
Sub endometrial stem cells |
Successful IVF conception |
|
4 |
Spp 33 / 8 |
Poor responder Very poor response to the oral and injectable ovulation agents |
Uterus Normal |
16x17x16 mm Vol 2.4 ml AFC= 1-2 |
17x20x21 mm Vol 4.11 ml AFC= 0- 2 |
Uterus Normal Both ovaries appears smaller atrophic |
Endometrial cavity normal Poor endometrial quality |
Endometrium is poor and took almost 6 months for endometrial preparation Finally endometrium was 7.3 mm thick with zone 5 vascularity IVF – FET done |
Intra ovarian stem cells
One blast formed |
IVF FET DONE FAILED |
|
5 |
MPG 35/13 |
Very poor response to the endometrial preparation Embryo are ready but endometrium is not well responding |
Uterus normal Thin endometrium |
Smaller in size atrophic |
Smaller in size atrophic |
Uterus Normal Both ovaries appears smaller atrophic |
Endometrial cavity normal Poor endometrial quality |
Embryos are ready but endometrium is not responding well Suggestive of poor endometrial response |
Intraovarian and sub endometrial stem cells |
Planning for endometrial preparation |
|
6 |
SPM 24/4 |
Poor responder |
Uterus rt unicornuate Poor responder AMH-0.8 FSH -8.5 |
UTERUS 31X23 MM UNICORNUATE |
ovary small atrophic |
ovary small atrophic |
ENDOMETRIUM IS GOOD |
Non responding well ovulation induction drugs Poor follicular respoce
|
Intraovarian Stem cells
Opu done 6 oocytes 3- M2 And 3 - abnormal oocytes
3 day 3 embryos |
FET done Positive |
|
7 |
SCG 29/7 |
Poor responder |
Uterus normal |
Smaller 26x17x20 mm |
Smaller 23x18x21 mm |
Uterus normal Both ovaries appears smaller atrophic |
Endometrial cavity good |
Non responding well ovulation induction drugs Poor follicular respoce
|
Intraovarian Stem cells
Opu done 3 oocytes 2- M2 And 1 - abnormal oocytes
1 day 3 embryos |
FET DONE |
|
8 |
Vsp 41/12 |
Poor responder Three abortions Two blighted ovum and one missed abortion |
Uterus bulky adenomyotic One anterior wall fibroid One posterior wall fibroid Fibroids non indentiong the endometrial cavity |
Smaller 18x16x20 mm |
Smaller 15x18x19 mm |
Uterus bulky adenomyotic Smaller atrophic ovaries |
Endometrial cavity appears normal |
Intraovarian stem cells planned |
3 oocytes 2 abnormal empty ooplasm One good m2 Failed fertilization |
|
|
9 |
MSS 25/5 |
Poor responder Premature ovarian failure AMH – 0.6 FSH – 9.7 |
UTERUS NORMAL |
Smaller AFC = 1-2 |
SMALLER AFC = 0-1 |
uterus slightly bulky ovaries smaller atropic |
endometrial cavity appears normal |
Non responding well ovulation induction drugs Poor follicular respoce
|
intraovarin stem cell opu done 3 m 2 oocytes One ady 3 good embryo |
FET Failed |
|
10 |
Ssp 37/17 |
Poor responder Severe adenomyotic uterus Kinked endometrial cavity Non responding endometrium |
Massive adenomyosis with kinked cavity poor endomyometrial differentiation Thin hazy non triple line endometrium with poor vascularity |
Smaller atrophic |
Smaller atrophic |
Severe adenomyoic bulky uterus with smaller atrophic ovaries |
Normal cavity capacity but poor endometrial quality |
Nonresponding , thin hazy , nontriple line , typical adenomyotic endometrial response |
Subendometrial stem cells |
FET DONE TWIN PREGNANCY DELIVERED BABY -1 – 2.5KG AND BABY -2 – 2.3KG |
Table 1: Table showing details of the synergetic effects of stem cells and PRP enhance and restoring various aspects of ovarian function, such as folliculogenesis, vascular formation, genetic stability and its clinical outcomes in various study subjects.
**Note: Atrophic is a term given to signify a structure that is shrunken or diminished in size and function. In the case of the ovary this implies a decreased in the ovarian tissue volume and may be indicative of ovarian failure or interrupted ovarian blood supply.
Conclusion
It is evident that combine effect of BMSCF and PRP therapies have potential in treating POD. The synergetic effects of stem cells and PRP enhance and restoring various aspects of ovarian function, such as folliculogenesis, vascular formation, and genetic stability. Since stem cells are a proven new therapeutic strategy for the future cure of ovarian dysfunction.
Acknowledgment
The author thanks to Dr. Sandeep Nemani, Haematologist for his support in getting patient’s bone marrow during this various studies of patients. Also we thank to all IVF staff of Dr Ghodake IVF and fertility centre, Sangli for their support.
References
- Aly RM (2020) Current state of stem cell-based therapies : an overview. Stem Cell Investig 7: 8.
- Mousaei Ghasroldasht M, Seok J, Park HS, Liakath Ali FB, Al-Hendy A (2022) Stem Cell Therapy : From Idea to Clinical Practice. Int J Mol Sci 23: 2850.
- Kharat A, Padmanabhan U, Gadre SV (2017) Effect of growth hormone–releasing hormone and IGF-1 on human placenta-derived mesenchymal stem cells’ proliferation and differentiation. J Stem Cells 12: 7-15.
- Polymeri A, Giannobile WV, Kaigler D (2016) Bone Marrow Stromal Stem Cells in Tissue Engineering and Regenerative Medicine. Horm Metab Res 48: 700-713.
- Kharat A, Chandravanshi B, Gadre S, Patil V, Bhonde R, et al. (2019) IGF-1 and somatocrinin trigger islet differentiation in human amniotic membrane derived mesenchymal stem cells. Life Sci 216: 287-294.
- Patil VR, Kharat AH, Kulkarni DG, Kheur SM, Bhonde RR (2018) Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol Int 42: 1-20.
- Torrealday S, Kodaman P, Pal L (2017) Premature Ovarian Insufficiency - an update on recent advances in understanding and management. F1000Res 6: 2069.
- Chon SJ, Umair Z, Yoon MS (2021) Premature Ovarian Insufficiency : Past , Present , and Future. Front Cell Dev Biol 9: 672890.
- Yang Z, Du X, Wang C, Zhang J, Liu C, et al. (2019) Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther 10: 250.
- de Miguel-Gómez L, López-Martínez S, Francés-Herrero E, Rodríguez-Eguren A, Pellicer A, et al. (2021) Stem Cells and the Endometrium : From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 10: 595.
- Nazaroff J, Oyadomari S, Brown N, Wang D (2021) Reporting in clinical studies on platelet-rich plasma therapy among all medical specialties: A systematic review of Level i and II studies. PLoS One 16: 0250007.
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L (2017) Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. J Bras Reprod Assist 21: 54-56.
- Tandulwadkar SR, Naralkar MV, Surana AD, Selvakarthick M, Kharat AH (2017) Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: A pilot study. J Hum Reprod Sci 10: 208-212.
- Samy A, Abbas AM, Elmoursi A, Elsayed M, Hussein RS (2020) Effect of autologous platelet-rich plasma transfusion in the treatment of infertile women with thin endometrium and its implications in IVF cycles: a literature review. Middle East Fertility Society Journal 25: 5.
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21: 7794.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017) Mesenchymal Stem Cell Secretome : Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci 18: 1852.
- Gupta S, Lodha P, Karthick MS, Tandulwadkar SR (2018) Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: Review of literature and a case report of world’s first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J Hum Reprod Sci 11: 125-130.
- Mansour RN, Soleimanifar F, Abazari MF, Torabinejad S, Ardeshirylajimi A, et al. (2018) Collagen coated electrospun polyethersulfon nanofibers improved insulin producing cells differentiation potential of human induced pluripotent stem cells. Artif Cells Nanomed Biotechnol 46: 734-739.
- Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, et al. (2018) Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders, Fertil Steril 110: 496-505.
- Green LJ, Zhou H, Padmanabhan V, Shikanov A (2019) Adipose-derived stem cells promote survival, growth, and maturation of early-stage murine follicles. Stem Cell Res Ther 10: 1-13.
- Tomaszewski CE , Constance E , Lemke MM , Zhou H , Padmanabhan V, et al. (2020) Adipose-derived stem cell-secreted factors promote early stage follicle development in a biomimetic matrix. Biomater Sci 7: 571-580.
Citation: Bansode M, Kore SA, Lee Y, Ghodake V, Verma R (2022) Clinical Approach of Novel Autologous Stem Cell Therapy for Primary Ovarian Insufficiency and Endometrial Regeneration. J Stem Cell Res Dev Ther 8: 102.
Copyright: © 2022 Manoj Bansode, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

