
Cobalt Syndrome Following Total Knee Arthroplasty: A Case-Based Review and Clinical Implications
*Corresponding Author(s):
Vaidya BalaMedical Co-Director, Public And Population Health, The Wollongong Hospital, Australia
Email:Vaidya.balasubramaniam@health.nsw.gov.au
Abstract
Background: Cobalt syndrome, or arthroprosthetic cobaltism, is a rare but serious complication of joint arthroplasty caused by systemic cobalt ion exposure. Traditionally linked to metal-on-metal hip implants, recent evidence implicates polyethylene liner degradation in total knee arthroplasty (TKA) as a source of metal ion release.
Objective: To present a case of unilateral cobalt syndrome following bilateral TKA and integrate current literature to highlight diagnostic and therapeutic considerations.
Methods: A comprehensive independent medical examination and literature review were conducted. Clinical findings were correlated with imaging, implant analysis, and manufacturer recall data.
Results: The patient developed chronic synovitis and systemic symptoms due to polyethylene liner degradation and metal-on-metal contact. Diagnostic workup supported cobaltism. Management included multidisciplinary pain care, cobalt assays, and recommendations for implant revision.
Conclusion: Cobalt syndrome should be considered in patients with unexplained symptoms post-TKA, especially when involving recalled implants. Early recognition and intervention are critical to prevent long-term morbidity.
Keywords
Adverse Local Tissue Reaction; Cobalt Syndrome; Exactech Recall; Metallosis; Polyethylene Liner Degradation; Rehabilitation; Total knee arthroplasty.
Introduction
Cobalt syndrome, also known as metallosis or adverse local tissue reaction (ALTR), is characterised by local inflammation and systemic toxicity due to cobalt ion release from orthopaedic implants [1]. While primarily associated with metal-on-metal hip arthroplasty, polyethylene liner degradation in TKA has emerged as a potential source of cobalt exposure [2]. The Exactech Optetrak Logic system was globally recalled in 2022 due to packaging faults that accelerated polyethylene oxidation [3]. This degradation can lead to micromotion, edge loading, and unintended metal-on-metal contact, triggering cobalt and chromium ion release [4].
Methods
This report synthesises findings from a detailed independent medical examination conducted in September 2025 of a 58-year-old male patient who underwent bilateral TKA in June 2021. Data were extracted from surgical records, imaging, pathology reports, and manufacturer documentation. A literature review was conducted using PubMed, MDPI, and JAMA Network Open to identify relevant studies on cobaltism in arthroplasty.
Results
Clinical Presentation: The patient developed persistent left knee pain, swelling, and synovitis 18 months post-TKA. Imaging revealed synovial inflammation without loosening. Aspiration ruled out infection. Systemic symptoms included fatigue, anxiety, impaired mobility, and suspected thyroid dysfunction (Table 1).
Implant Composition:
|
Component |
Material |
Clinical Relevance |
|
Femoral Component |
Cobalt-Chromium-Molybdenum Alloy |
Source of cobalt and chromium ions |
|
Tibial Baseplate |
Titanium Alloy |
Generally inert, low ion release |
|
Tibial Insert |
Ultra-High Molecular Weight Polyethylene |
Subject to oxidative degradation and wear |
Table 1: Degradation of polyethylene may lead to metal-on-metal contact between femoral and tibial components.
Pathophysiological Cascade
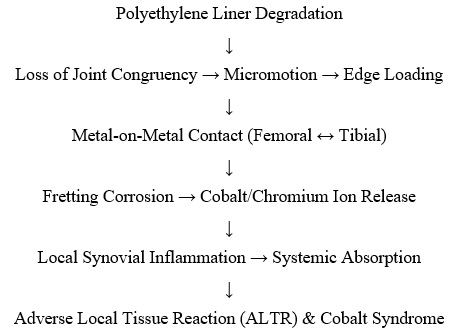
Investigations and Management:
Physical examination revealed a warm, swollen left knee with limited flexion and instability. Imaging confirmed synovitis without loosening. Cobalt assays and thyroid function tests were performed. Revision surgery was planned, and interim management included multidisciplinary rehabilitation and pain care.
Discussion
This case underscores the diagnostic complexity of cobalt syndrome in TKA. Although knee implants are not typically associated with metal-on-metal articulation, polyethylene degradation can result in unintended metal contact and ion release [5]. Studies show that 57% of patients with cobalt-chrome implants test cobalt-positive, with systemic symptoms such as fatigue and thyroid dysfunction [6]. The European Union’s classification of cobalt as a carcinogen highlights the need for long-term surveillance [7].
Hypoallergenic revision strategies have been described for metal allergy in knee arthroplasty [8]. Vigilant surveillance following device recalls, including metal ion quantification and targeted imaging, is crucial for early intervention [9].
Conclusion
Cobalt syndrome is a clinically significant but underrecognized complication of TKA. This case demonstrates the importance of early diagnosis and intervention, particularly in the context of recalled implants. Multidisciplinary care and surgical revision are essential for symptom resolution and functional recovery.
Acknowledgement
I want to acknowledge the law firm Commins Hendriks, located at 23 Gurwood Street, Wagga Wagga, NSW, Australia 2650, and the client who attended for an assessment for a medical negligence claim, who made it possible to further research and investigate this clinical disorder.
Funding Statement
This research received no external funding.
Conflict of Interest
The author declares no conflict of interest related to this manuscript.
Ethical Approval
This case report was prepared in accordance with ethical standards. Patient consent was obtained for publication.
References
- Jacobs JJ, Hallab NJ, Urban RM, Wimmer MA (2006) Wear particles. J Bone Joint Surg Am 2: 99-102
- Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, et al. (2014) Risk stratification algorithm for management of patients with dual modular taper total hip arthroplasty. consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons and the Hip Society. J Arthroplasty 29: 2060-4.
- Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, et al. (2005) Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am 87: 28–36.
- Thomas P, von der Helm C, Schopf C (2021) Metal allergy in total knee arthroplasty: Revision surgery using a hypoallergenic implant in 11 patients. Journal of Orthopaedic Surgery and Research 16: 1–9.
- Exactech Inc (2022) Urgent Medical Device Correction - Optetrak Logic Polyethylene Inserts.
- Tower SS, Cho CS, Bridges LR, Gessner BD (2021) Prevalence of Cobalturia Among Adults With Joint Replacements. JAMA Network Open. 4: 2121758.
- Tower SS, Gessner BD, Cho CS, Bridges L (2023) The association of cobalturia with cobaltism symptoms a prospective blinded study of 229 post-arthroplasty patients. PLoS One 18: 0295203
- Brüggemann A, Hailer NP (2024) Concentrations of Cobalt, Chromium and Titanium and Immunological Changes after Primary Total Knee Arthroplasty—A Cohort Study with an 18-Year Follow-Up. J. Clin. Med 13: 951
- American Academy of Orthopaedic Surgeons (2024) Updated clinical practice guideline for the prevention of total hip and knee arthroplasty periprosthetic joint infection in Patients Undergoing Dental Procedures. AAOS Newsroom.
Citation: Bala V (2025) Cobalt Syndrome Following Total Knee Arthroplasty: A Case-Based Review and Clinical Implications. J Orthop Res Physiother 11: 063
Copyright: © 2025 Vaidya Bala, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

