
Comparative Study of the Efficient Growth Medium for Screening Phosphate Solubilizing Pseudomonas with ACC-Deaminase Activity
*Corresponding Author(s):
Amina MelianiResearch Laboratory In Geo Environment And Spaces Development, Faculty Of Natural And Life Sciences, Mustapha Stambouli University, BP 763, 29000 Mascara, Algeria
Email:amina.meliani@univ-mascara.dz
Abstract
The use of phosphate solubilising Pseudomonas as inoculants has provided an alternative biotechnological solution in sustainable agriculture to meet the P and N2 demands of plants. Phosphate Solubilizing Bacteria (PSB) possessing the ability to solubilize insoluble phosphate was isolated from the rhizospheric soil of Triticum sp. Of the eight bacterial isolated, five were able to produce siderophores and to solubilize phosphate both in Pikovskaya (PVK) and National Botanical Research Institute's phosphate growth (NBRIP) medium. The highest phosphate solubilization efficiency was signaled in NBRIP medium for P.putida (Ps6) isolate (22 mm) followed by P.fluorescens (Ps11) isolates (17mm) vis a vis 19mm and 16 mm in PVK medium, respectively. Furthermore, the results indicated that only three isolates were able to cleave ACC into α ketobutyrate and ammonia with a low degrees of efficacy (OD550<0.5). Thus, phosphate solubilization activity may be useful criteria for the selection of effective plant growth promoting rhizobacteria to be used as biofertilizers.
Keywords
Bio fertilizers; Phosphate; Pseudomonas; Selection; Solubilizing
Introduction
To produce more food, the world will require (at least initially) more agricultural land; greater use of chemicals including fertilizers, pesticides and herbicides; more farm mechanization; greater use of transgenic crops; and the expanded use of plant growth-promoting microorganisms. Of course, many of the solutions to this problem that will be attempted are not sustainable and will only be effective in the short term. Since we live in a finite world with limited resources, any effective and longer term solutions to providing food for the world (once the population has leveled off) must include sustainable and environmentally friendly biological solutions To this end, the purposeful use of PGPB in agriculture is an attractive technology to address this problem [1].
The large-scale application of PGPR to crop as bio fertilizers would be attractive as it would substantially reduce the use of chemical fertilizers and pesticides, which often pollute the environment. This has a heavy impact on the natural and human environment, as well as on human health, through the pollution of soils, waters, and the whole food supply chain. The PGPR can promote growth of several types of plants [2]. Furthermore, stimulation of different crops by PGPR has been demonstrated in both laboratory and field trials. Strains of Pseudomonas putida and Pseudomonas fluorescens have increased root and shoot elongation in canola, lettuce, and tomato [3] as well as crop yields in potato, radishes, rice, sugar beet, tomato, lettuce, apple, citrus, beans, ornamental plants, and wheat [4,5].
Generally, the mechanisms by which PGPR can exert a positive effect on plant growth can be of two types: direct and indirect [6,7]. Indirect growth promotion is the decrease or prevention of deleterious effect of pathogenic microorganisms [8], mostly due to the synthesis of antibiotics [9] and siderophores [10]. Whereas the direct growth promotion can be through the synthesis of phytohormones [11], N2 fixation [11], the uptake of certain nutrients from the environment [12] and synthesis of some enzymes such as ACC deaminase 1-(aminocyclopropane-1-carboxylate deaminase) that modulate the level of plant hormones [13], as well as the solubilization of inorganic phosphate and mineralization of organic phosphate, which makes phosphorous available to the plants [1]. The main objective of the present study was to establish a procedure for the identification of efficient phosphate solubilizer from soil. Our scope deals the phosphate solubilization and ACC deaminase of the mentioned species (P. putida and P. fluorescens).
Materials and Methods
Isolation and biochemical characterization of Pseudomonas fluorescents (PGPR)
The biochemical characterization was determined by means of API 20NE; bio Merieux Vitek strips and on the basis of Pseudomonas biochemical tests as described in Bergy’s Manual of Determinative Bacteriology.
Each isolate was tested for morphology, motility and Gram stain. The following physiological tests were performed: fluorescent and pigment production, accumulation of levan production, oxidative or fermentative acid production, growth at 4 and 41°C. The enzymatic activities tested were: lipase production, pectinolytic activity, starch hydrolysis, lecithinase production and proteolytic enzyme production.
Most of the tests conducted for their identification have been based on physiological, nutritional tests [14] and by the use of the Analytical Profile Index (API 20NE; bio Merieux Vitek), strains were maintained in LB at -80°C with 50 per cent glycerol.
Solubilization of phosphate
Bacterial strains were tested by plate assay using Pikovskaya (PVK) medium (in l-1: glucose,10 g; Ca3(PO4)2, 5 g; (NH4)2SO4, 0.5 g; NaCl, 0.2g; MgSO4-7H2O, 0.1 g; KCl, 0.2 g; yeast extract, 0.5g; MnSO4-H2O, 0.002 g; and FeSO4-7H2O, 0.002 g) and National Botanical Research Institute's phosphate growth (NBRIP) medium (in l-1: glucose, 10 g: Ca3(PO4)2, 5 g; MgCl2-6H2O, 5 g; MgSO4-7H2O, 0.25 g; KCl, 0.2 g and (NH4)2SO4, 0.1 g. A total of eight strains were tested by plate assay using PVK and NBRIP media supplemented with 1.5% Bacto-agar. Each strain was stabbed in triplicate using sterile toothpicks. The halo and colony diameters were measured after 18 days of the incubation of plates at 28 °C. Halo size was calculated by strains per plate were stabbed in triplicate using sterile toothpicks. The halo and colony diameters were measured after 14 days of the incubation of plates at 28 °C.
Halo size (h) was determined by subtracting the diameter of the colony from the diameter of the halo. According [15] to isolates were grouped into 5 classes according to their h score; class 0: lack of halo, class 1: h = 0–2 mm, class 2: h = 2–4 mm, class 3: h > 4 and class 4: h > 4 and a perfectly transparent halo. Class 4 includes a qualitative aspect of the halo, as halos of class 3 were as large as class 4 but rather diffuse.
Aminocyclopropane-1-carboxylic acid deaminase (ACC) assay
ACC-metabolism assay (qualitative) was carried out to characterize the rhizobacterial strains for their ability to use ACC as a sole nitrogen source. The rhizobacterial strains were grown on two nitrogen sources (ACC and ammonium sulphate) and one mineral source (magnesium sulphate), to observe the growth rate of the strain, for ACC substrate in parallel to ammonium sulphate. These strains were categorized into three groups, as strains with higher (OD550> 0.7), medium (OD550: 0.5-0.69) and lower (OD550< 0.5) ACC-metabolism rate depending upon their O.D value at 550 nm for ACC substrate as compared to ammonium sulphate. ACC-metabolism assay was proceeded according to the method (modified) described by [16].
Quantitative ACC deaminase activity was assayed according to the method of [17] with some modifications, which measures the amount of α-ketobutyrate produced when the enzyme ACC deaminase cleaves ACC. The quantity of nmol of α-ketobutyrate produced by this reaction was determined by comparing the absorbance at 540 nm of a sample to a standard curve of α-ketobutyrate ranging between 0·1 and 1·0 nmol. A stock solution of 100 mmol l−1α-ketobutyrate (Prochima-Sigma, ref: K401-10) was prepared in 0·1 mol l−1 Tris–HCl (pH 8·5) and stored at 4°C. Just prior to use, the stock solution is diluted with the same buffer to make a 10 mmol l−1 solution from which a standard concentration curve is generated. Each in a series of known α-ketobutyrate concentrations is prepared in a volume of 200 μl and 300 μl of the 2,4-dinitrophenyl hydrazine reagent (0·2% 2,4-dinitrophenyl hydrazine in 2 mol l−1 HCl) was added, and the contents were vortexed and incubated at 30°C for 30 min. The colour was developed by the addition of 2·0 ml 2 mol l−1 NaOH and the readings were taken at 540 nm.
Production of ammonia
The different isolates of Pseudomonas sp. (PGPR) were grown in peptone water in tubes and were incubated at 30°C for 4 days. 1 ml Nessler’s reagent was added in each tube. Tubes were observed for presence of a yellow to brownish colour for maximum production of ammonia [18].
Siderophore production
Siderophore production was detected according to [19] study. The bacterial cultures were streaked on the King’s B medium with and without (50 mg/l) FeCl3 and incubated at 28 ± 2°C for 48 h. Fluorescent pigment formed was considered as an indication of siderophore production. Further qualitative production of siderophore was tested using Chrome Azurol S (CAS) agar [20]. Presence of siderophore production was indicated by orange halos around the colony due to chelation of iron which bound to CAS dye.
Statistical Analysis
The statistical processing of the data obtained from all studies was implemented by means of dispersion analysis with the STATISTICA 7 software. Data are expressed as means ± standard deviation (SD). Statistical analysis was performed with an analysis of variance (ANOVA) and correlation, a difference was considered statistically significant when p ≤ 0.05.
Results And Discussion
Among the eight isolates (P.putida (Ps6, Ps10) and P.fluorescens (Ps2, Ps4, Ps11, P4, P9, P10)), five were phosphatase positive, and have shown positive result for ammonia and siderophores production. However only three isolates had variable ACC-deaminase activity.
Phosphate solubilization
Eight isolates were tested for semi-quantitative phosphate solubilization activity; most of the isolates (5) were classified as class 4, whereas 3 isolates showed no apparent halo (Figure.1). Undoubtedly, the medium used to test phosphate solubilization activity of selected PSB indicated that isolates can grow on glucose as sole carbon source and solubilize Ca3 (PO4)2. On the basis of halo observations and measurements in medium, glucose and Ca3 (PO4)2 seem to be essential for phosphate solubilization whereas the yeast extract is a nonessential component of the medium. These findings are consistent with [21] report, the amount of glucose as a carbon source played an important role in the phosphate solubilization. Moreover, the rate of the phosphate solubilization was increased with increasing concentrations of glucose.
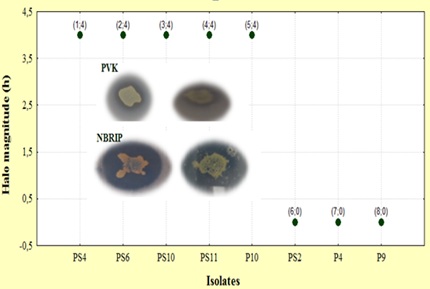 Figure 1: Class of the semi quantitative phosphate solubilization assay on the basis of halo magnitude.
Figure 1: Class of the semi quantitative phosphate solubilization assay on the basis of halo magnitude.
Phosphate solubilization ability of Pseudomonas sp. 2 increased by about 30% in the absence of either yeast extract or (NH4)2SO4. In the absence of both yeast extract and (NH4)2SO4, the phosphate solubilization ability of Pseudomonas sp. 2 was enhanced by 12.5%. It was interesting to note that by simply omitting yeast extract from PVK consistently higher phosphate solubilization levels were obtained [21]. This further proved that the presence of yeast extract in the PVK medium was inhibitory to the phosphate solubilization compared with NBRIP medium.
Comparative studies on NBRIP and PVK with eight bacteria in a plate assay showed different results, however, in NBRIP, the P solubilization was more efficient compared to PVK medium for all the five strains. Efficiency of phosphate solubilization by the strain Ps6 and Ps11 in NBRIP was significantly higher compared to PVK with a diameter of 22 mm,17mm vis a vis of 19mm and 16 mm, respectively. The ability of the strains to solubilize phosphorus in both media was also maintained at a higher level throughout the duration of 18 days (Figure2 and Figure3). This further augments well for the use of NBRIP as an efficient phosphate solubilization medium over PVK.
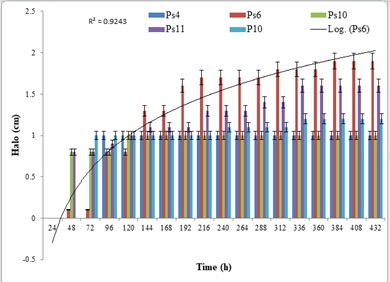 Figure 2: Halo size (h) of phosphate solubilization on Pikovskaya (PVK).
Figure 2: Halo size (h) of phosphate solubilization on Pikovskaya (PVK).
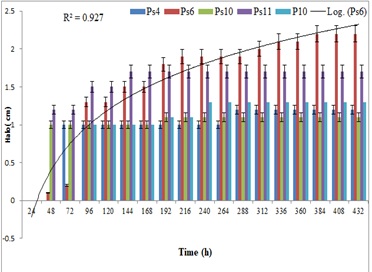 Figure 3: Halo size of phosphate solubilization on National Botanical Research Institute's phosphate growth medium (NBRIP).
Figure 3: Halo size of phosphate solubilization on National Botanical Research Institute's phosphate growth medium (NBRIP).
The physiology of phosphate solubilization has not been studied thoroughly. Some studies indicate that certain mineral elements play a role in this process. A critical K concentration is necessary for optimum solubilization rates, while Mg and Na seem to be important in some fungi but not in Pseudomonas strains. The role of N and P uptake remains controversial [22].
Our results are in accordance with those of other studies such as [23] and [21], who found that NBRIP is more efficient than PVK medium. Pseudomonas spp. and Bacillus spp. were found to have more phosphate solubilizing capacity than the other genera (Vibrio, Alcaligens and Corynebacterium). On plate assays, Pseudomonas showed larger zones of solubilization indicating an efficient solubilization of insoluble and fixed phosphates than the other strains [24]. [25] While studying the Porto Novo waters reported Pseudomonas spp. and Bacillus spp. as dominant inorganic phosphorus compounds solubilizing microbes.
Phosphate Solubilising Bacteria (PSB) has provided an alternative biotechnological solution in sustainable agriculture to meet the P demands of plants. These organisms in addition to providing P to plants also facilitate plant growth by other mechanisms. Current developments in our understanding of the functional diversity, rhizosphere colonizing ability, mode of actions and judicious application are likely to facilitate their use as reliable components in the management of sustainable agricultural systems [26]. PSM include largely bacteria and fungi. The most efficient PSM belong to genera Bacillus, Rhizobium and Pseudomonas amongst bacteria, and Aspergillus and Penicillium amongst fungi [27]. According to [28] the fluorescents Pseudomonas are known as good phosphate solubilizers against Azotobacter and the Rhizobia species”.
The colonies that had higher solubilization zones (selected PSB) were purified and screened based either on the solubilization index on Pikovskaya and NBRIP agar for ACC deaminase activity.
ACC-deaminase activity
Qualitative results of ACC assay revealed that only PS4, PS11 and PS10 strains metabolized ACC into α ketobutyrate and ammonia with a low degrees of efficacy (OD550<0.5). PS4, PS11 and PS10 showed a low amount of ACC deaminase activity (105, 85, 41 nmol h-1 ACC-deaminase activity, respectively) where in other strains no activity was recorded.
ACC deaminase has been found in a wide range of Gram-negative [29] and Gram-positive bacteria [30]. In certain PGPR the enzyme ACC deaminase cleaves ACC to form α-ketobutyrate and ammonium and thereby lowers the level of ethylene in developing or stressed plants [16]. The PGPR containing ACC deaminases are present in various soils and offer promise as a bacterial inoculum for improvement of plant growth. When ACC deaminase-containing PGPR colonize the rhizosphere, they can act as a sink for ACC and keep ethylene levels below the point where root growth is impaired [6]. Such activity is important during normal plant development and also protects plants from the deleterious effects of environmental stresses, including phytopathogens, flooding [31], drought [32], and heavy metals [30].
Ethylene is an important phytohormone, but over-produced ethylene under stressful conditions can result in the inhibition of plant growth or death, especially for seedlings. PGPR containing ACC deaminase can hydrolyze ACC, the immediate precursor of ethylene, to α-ketobutarate and ammonia, and in this way promote plant growth. Inoculation of crops with ACC deaminase-containing PGPR may assist plant growth by alleviating deleterious effects of salt stress ethylene [30].
Production of ammonia
Out of the five isolates tested, PS4, PS11, PS10 and P10 were found to be ammonia producers. These results are in close agreement with those of Joseph et al. (2007) who revealed the production of ammonia commonly detected in the isolates of Bacillus (95%) followed by Pseudomonas (94.2%), Rhizobium (74.2%) and Azotobacter (45%). Similarly, [33] isolated the Pseudomonas putida from the rhizosphere of Pisum sativum. The organism exhibited a battery of PGPR characteristics and was also found positive for the production of ammonia.
Development of yellow-brown color was observed after addition of Nessler’s reagent indicating a positive test for ammonia production. It has been reported that ammonia production indirectly influences the plant growth. Pseudomonas fluorescens strain MA-4 was efficient in ammonia production and significantly increased biomass of medicinal and aromatic plant such as Geranium [34]. Ammonia production was detected in 95% of the isolates from the rhizosphere of rice, mangrove and effluent contaminated soil influencing plant growth promotion [35].
Siderophore production
Results revealed that five isolates produced siderophores, the highest rate 53.6µM and 53.3µM was recorded for P4 and P9, respectively. Distribution of siderophore-producing isolates according to amplified ribosomal DNA restriction analysis (ARDRA) groups, reveals that most of the isolates belong to Gram- negative bacteria corresponding to the Pseudomonas and Enterobacter genera and Bacillus and Rhodococcus genera are the Gram-positive bacteria found to produce siderophores [36].The two strains Fluorescent Pseudomonas and Pseudomonas fluorescens NCIM 5096 along with P. putida NCIM 2847 produce maximum yield of hydroxamate type of siderophore in the modified succinic acid medium (SM) [37].
Statistically and in order to confirm the correlation between phosphate solubilization and the other PGPR traits we performed a regression analysis for the screened strains, it appears that there is no a significant relationship between the phosphatase activity, ACC deaminase and siderophores production. Although we found a significantly positive relationship (r2= 0.98 and P= 0.001) for phosphate solubilization in both medium. Even though the correlations obtained in the present study are not statistically significant, this disparity can be attributed to the direct and independent action of the PGPR strains. According to there are numerous Phosphate Solubilizing (PS) bacteria that possess the ability to synthesize a key enzyme, 1-aminocyclopropane-1-carboxylate (ACC) deaminase.
Conlusion
The use of biofertilizer such as N2 (nitrogen) fixing and phosphate solubilizing bacteria (PSB) can reduce chemical fertilizer applications and consequently lower reduction cost. The use of PSB as biofertilizers PGPR in order to increase the productivity may be a viable alternative to organic fertilizers and could decrease the environmental problems associated with conventional chemical fertilizers, which also helps in reducing the pollution and preserving the environment in the spirit of an ecological agriculture. In addition to biological nitrogen fixation, phosphate solubilization is very important in enhancing the soil fertility. PSB play an important role in supplying phosphate to plants, which is environment-friendly and sustainable approach. Further study is required in this area to gain a more accurate understanding of the potential agricultural benefits of Pseudomonas-mediated biofertilization.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The research was supported by the national research project (PNR project) of the Direction for Research Programming, Evaluation and Prospective Study (DGRSDT), Government of Algeria. The anonymous reviewers are sincerely thanked for their beneficial suggestions to improve the manuscript.
References
- Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to fee the world. Microbiological Research 169: 30-39.
- Piromyou P, Buranabanyat B, Tantasawat P, Tittabutr P, Boonkerd N, et al. (2011) Effect of plant growth promoting rhizobacteria (PGPR) inoculation on microbial community structure in rhizosphere of forage corn cultivated in Thailand. Eur J of Soil Biology 47: 44-54.
- Glick BR, Changping L, Sibdas G, Dumbroff EB (1997) Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biol Biochem 29: 1233-1239.
- Kloepper JW (1994) Plant growth promoting bacteria (other systems). In: Okon J, editor. Azospirillum/Plant As sociation. Boca Raton, FL: CRC Press 137-154.
- Kloepper JW, Lifshitz K, Schroth MN (1988) Pseudomonas inoculants to benefit plant production. ISI Atlas Sci Anim Plant Sci 60-64.
- Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41: 109-117.
- Glick BR (2001) Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21: 383- 393.
- Raj SN, Chaluvaraju G, Amruthesh KN, Shetty HS, Reddy MS, et al. (2007) Induction of growth promotion and resistance against downy mildew on pearl millet (Penninsetum glaucum) by rhizobacteria. Plant Dis 87: 380-384.
- Sivan A, Chet I (1992) Microbial control of plant diseases. In: Mitchell R, editor. Environmental Microbiology. New York: Wiley-Liss 335-354.
- Leong J (1986) Siderophores: Their biochemistry and possible role in the biocontrol of plant pathogens. Annu RevPhytopathol 24: 187-208.
- Glick BR (2012) Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientica 15.
- Çakmakçi R, Dönmez F, Aydm A, Sahin F (2006) Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol Biochem 38: 1482-1487.
- Duca D, Lorv J, Patten CL, Rose D, Glick BR, et al. (2014) Indole-3-acetic acid in plant- microbe interactions. Antonie van Leeuwenhoek 106: 85-125.
- Palleroni NJ (1986) Taxonomy of the pseudomonads. In: Sokatch, J.R. (Ed.), The Bacteria (The Biology of Pseudomonads). Academic Press Inc., Orlando, FL 3-25.
- Azziz G, Bajsa N, Haghjou T, Taulé C, Valverde A, et al. (2011) An abundance, diversity and prospecting of culturable phosphate solubilizing bacteria on soils under crop–pasture rotations in a no-tillage regime in Uruguay. Appl Soil Ecol 10: 3-7.
- Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and haracterization of 1 aminocyclopropane 1carboxylate deaminase from the plant growth promoting rhi-zobacterium Pseudomonas Can J Microbiol 40: 1019-1025.
- Honma M, Shimomura T (1978) Metabolism of 1 ami nocyclopropane 1 carboxylic acid. Agric Biol Chem 42: 1825-1831.
- Cappuccino JC, Sherman N (1992) In Microbiology A Laboratory Manual New York. 125-179.
- Teintze M, Hossain MB, Baines CL, Leong J, Van der Helm D (1981) Structure of ferric pseudobactin a siderophore from a plant growth promoting Pseudomonas. Biochem20: 6446-6457.
- Schwyn B, Neilands JB (1987) Universal chemical assay for detection and determination of siderophores. Anal Biochem160: 47-56.
- Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters 170: 265-270.
- Cabala-Rosand P, Wild A (1982) Direct use of low grade phosphate rock from Brazil as fertilizer II. Effects of mycorrhiza inoculation and nitrogen source. Plant Soil 65: 363-373.
- Rosas SB, Andrés JA, Rovera M, Correa NS (2006) Phosphate solubilizing Pseudomonas putidacan influence the rhizobia legume symbiosis. Soil Biol Biochem 38: 3502-3505.
- Seshadri S, Ignacimuthu S (2002) Variations in hetetrophic and phosphate solubilizing bacteria from Chennai southeast coast of India. Indian Journal of Marine Sciences 31: 69-72.
- Venkateswaran K, Natarajan R (1983) Seasonal distribution of in-organic phosphate solubilizing bacteria and phosphatase producing bacteria in Porto Novo waters. Indian J Mar Sci12: 213-217.
- Zaidi Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiologica et Immunologica Hungarica56 : 263-284.
- Rivas R, Peix A, Mateos PF, Trujillo ME, Martinez-Molina E, et al. (2006) Biodiversity of populations of phosphate solubilizing rhizobia that nodulates chickpea in different spanish soils. Plant and soil287: 23-33.
- Browne P, Rice O, Simon MH, Burke J, Dowling DN et al. (2009) Superior inorganic phosphate solubilization is linked to phylogeny within the Pseudomonas fluorescensAppl Soil Ecol 43: 131-138.
- Ma W, Sebestianova SB, Sebestian J, Burd GI, Guinel FC, et al. (2003) Prevalence of 1 amino cyclopropane 1 carboxylate deaminase in Rhizobium spp. Antonie Van Leeuwenhoek 83: 285-291.
- Belimov A, Safronova A, Sergeyeva VI, Egorova TA, Matveyeva TN, et al. (2001) Characterisation of plant growth promoting rhizobacteria isolated from polluted soils and containing 1 aminocyclopropane 1 carboxylate deaminase. Can J Microbiol 47: 642-652.
- Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39: 11-17.
- Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci166: 525-530.
- Chacko S, Ramteke PW, John SA (2009) Amidase from plant growth promoting rhizobacterium. J Bact Res1: 46-50.
- Mishra R, Prakash KO, Alam M, Dikshit A (2010) Influence of plant growth promoting rhizobacteria (PGPR) on the productivity of Pelargonium graveolens Herit. Recent Research in Science and Technology 2: 53-57.
- Samuel S, Muthukkaruppan SM (2011) Characterization of plant growth promoting rhizobacteria and fungi associated with rice, mangrove and effluent contaminated soil. Current Botany2: 22-25.
- Tian F, Ding Y, Zhu H, Yao L, Du B (2009) Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Brazilian Journal of Microbiology40: 276-284.
- Saharan BS, Nehra V (2011) Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sciences and Medicine Research LSMR-21.
- Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol190: 63-68.
- Glick BR, Todorovic BJ, Czarny J, Cheng Z, Duan J et al. (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci26: 227-242.
- Joseph B, Patra RR, Lawrence R (2007) Characterization of plant growth promoting rhizobaacteria associated with chickpea (Cicer arientinum L.) Int J Plant Production 2: 141-152.
Citation: Meliani A, Nair S, Fatima D (2022) Comparative Study of the Efficient Growth Medium for Screening Phosphate Solubilizing Pseudomonas with ACC-Deaminase Activity. J Environ Sci Curr Res 5: 031.
Copyright: © 2022 Amina Meliani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

