
Comparison of the SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Assay and Direct Binding ELISA (S-IgG) with the Cytopathic Effect Assay (CPE) in Analyzing the Neutralization Antibody of Vaccination People
*Corresponding Author(s):
Xiiang YangLeide Biosciences Co., Ltd., China
Email:yangx@leidebio.com
Jie Wu
The Guangdong Provincial Center For Disease Control And Prevention, Guangzhou, Guangdong, China
Email:771276998@qq.com
Jiianrong Lou
Leide Biosciences Co., Ltd., China
Email:loujr@leidebio.com
Abstract
The safety and effectiveness of the COVID19 vaccine are the key in vaccine development. Due to the difficulty and dangerous of virus neutralization experiment, many replacement assays were developed for testing neutralization antibody. In this report, two kits that based on the competition assay (cPass sVAT) and based on direct antigen binding assay(S-IgG) were compared. The positive rate of cPass sVAT kit and S-IgG assay was 72.9% and 84.2% respectively in 59 sample of vaccinators. Both kit’s specificities reached 100% in 46 health control samples. Quantity analysis of the S-IgG results found that 94.9% (56/59) of vaccinators have produced neutralizing antibodies. The total coincidence rate between cPass sVAT and CPE was 86%, similar to the coincidence rate between S-IgG and CPE, which was 84.2%. S-IgG is relatively more sensitive and easier in quantitating the neutralization antibodies than the other two experiments.
Keywords
Covid19; cPass sVNT assay; S-IgG assay; Cytopathic Effect assay (CPE); Neutralization antibody
Introduction
SARS-CoV-2 has already infected over 124 million people around the world and killed more than 2.5 million people. The rapid development of the COVID-19 vaccines has brought hope to the control of this epidemic. There are currently more than 214 COVID-19 candidate vaccines in development worldwide [1,2]. Currently licensed vaccines include the mRNA vaccines (mRNA-1273 and BNT162b1) from Moderna and Pfizer/BioNTech (approved by the FDA of USA); adenovirus vaccine (Ad5-nCoV) from CanSino Biologics, the BBIBP-CorV from Sinopharm and CoronaVac from Sinovac (Approved by the NMPA of China), and AZD1222 from Oxford-AstraZeneca (Approved by MHRA of UK; the EMA of EU; the TGA of Australia et al). In the period of vaccine introduction, one of the main objectives of surveillance is to assess the effect of vaccine. The traditional approach to the evaluation of vaccine efficacy is to compare the incidence of infection among immunized subjects vs. unimmunized controls [3]. There are three approved vaccines in China belong to the inactivated vaccines [2]. The biggest concern with the inactivated vaccines is that they normally produced fewer neutralizing antibodies compared with the mRNA vaccines [2]. UAE approves Sinopharm vaccine for use after trials show 86% efficacy [4]. Although vaccine licensure requires evidence of vaccine safety and efficacy from randomized controlled trials (RCT), many questions about vaccine effectiveness (VE) can be answered only by observational approaches after the vaccine is in use [5].
Neutralization antibody is essential for the evaluation of vaccine effectiveness. When an antibody effectively prevents the virus from infecting the cell, the antibody is the so-called "neutralizing antibody" [6]. Neutralizing assay includes plaque reduction neutralization test (PRNT), cytopathic effect assay (CPE), competition ELISA assay, pseudo-virus neutralization assay and antigen-antibody indirect agglutination inhibition test, etc. [7-10]. The PRNT and CPE are methods to determine the ability of the immune serum to neutralize the virus based on the comparison of the residual infectivity of the virus after neutralization [11]. Although PRNT is the standard method recommended by WHO, it is time-costing, expensive and dangerous. The Duke University School of Medicine in the United States and the National University of Singapore Duke-NUS jointly developed a neutralizing antibody detection reagent based on a competitive ELISA. Based on the specific protein binding inhibition between the virus surface protein (RBD domain of spike protein) and the human receptor protein (ACE-2), the cPass™ sVNT Kit was developed [12,13]. It is claimed to have a sensitivity of 95%-100% and a specificity of 99.93% in clinical verification. Another ELISA-based S-IgG neutralizing antibody detection kit was developed by Leide Biosciences, which claimed to have a sensitivity of 93%, and 96% consistent with the PRNT method [14]. These two assays were developed based on the infected patients. Will they also work in evaluating vaccination efficiency?
Materials and Methods
Nunc 96-well microtiter Maxisorb plates were from Corning, USA. The microplate reader was SpectraMax 3 from Molecular Devices Inc. Skimmed milk was from Sangon Biotech, China. Mouse anti-human IgG horseradish peroxidase (HRP) conjugate was from Fapon, China. SARS-CoV-Surrogate Virus Neutralization kit (cPass sVAT) was from GenScript (Catalog No. L00847A).
Recruitment of patients and specimen collection
A total of 59 blood samples (age 21 to 53) were collected at 7 days after the second dose of inactive vaccine (BBIBP-CorV from Sinopharm) injected healthy people. And 46 blood samples (age 22 to 60) were collected from no SARS-COV2 virus exposure history, not vaccinated, healthy people. Blood plasm were collected after centrifuge at 3000 rpm for 10 minutes.
COVID-19 S-IgG ELISA kit was provided by Leide Biosciences. (#2022-96).
S protein was expressed in 293T cells and purified by Ni-sepharose. The purified S protein-based ELISA kits were used for the detection of IgG antibody against SARS-CoV-2 S protein. Total 5ul serum samples were diluted to 500ul and 100ul were used for test.
Vero-E6 cell line purchased from ATCC (CRL-1586).
Wild type SARS-COV2 virus come from Guangdong CDC (Gisaid library code is EPI_ISL_403934. Named as 20SF014/vero-E6/3. Plate reader iMark is from Bio-Rad.
Neutralization experiment
Stock virus was amplified by grown in Vero E6 cells. The virus stock was titrated by serial dilute by 10-fold. A confluent monolayer of Vero E6 cells was infected with 100 µl of each dilution in quadruplicate in 96-well plates. Cytopathic effect (CPE) was observed under microscope five days after inoculation. The endpoint dilution leading to CPE in 50% of inoculated wells was designated as one 50% tissue culture infecting dose (TCID50).
Serial four-fold dilutions of heat-inactivated sera were made. The serum dilutions 240ul were mixed with equal volumes of 100 TCID50 of SARS-CoV-2 as indicated. After 2 h of incubation at 37°C, 5%CO2 incubator, 100 μL of the virus–serum mixture was added in quadruplicate to Vero E6 cell monolayers in 96-well microtiter plates. Then, an additional 100 μL of culture medium IMM was added to each well and the plates incubated for 5 days at 37°C in 5% CO2 in a humidified incubator. A serial 10-fold dilution of the virus: 100 TCID50/50ul, 10 TCID50/50ul, 1 TCID50/50ul and 0.1 TCID50/50ul was made as control and loading into 8 wells of 96-well microtiter plates with additional 150ul of culture medium MM. The CPE was read at 5 days post infection. The highest serum dilution that completely protected the cells from CPE in half of the wells was taken as the neutralizing antibody titer. More than 4 times dilution could protect the cells from virus infection was set as the cutoff of the positive. These procedures were carried in a biosafety level 3 facility.
COVID-19 S-IgG ElISA assay
Each serum or plasma sample was tested at a dilution of 1:100 in sample dilution ELISA buffer (Supplied with each ELISA kits) and added to the ELISA wells of each plate for 1 hour shaking with 250rpm at room temperature. After 5 times washing with washing buffer, horseradish peroxidase (HRP)-conjugated goat anti-human IgG was added for 30 minutes at shaking with 250rpm at room temperature. The ELISA plates were then washed five times with washing buffer. Subsequently, 100 μL of HRP substrate was added into each well. After 15 min incubation, the reaction was stopped by adding 50 μL of stop solution and read optical density (OD) within 15 minutes at 450nm and 630nm on iMark microplate reader (Bio-Rad). Comparing the sample OD value with the Reference Control’s OD value, it is positive when the S/CO is greater than 1.
cPass™ sVNT Assay
Assay were done based on the assay manual. Briefly, Dilute HRP conjugated RBD with a 1:1000 dilution ratio with RBD Dilution Buffer. Dilute 10ul test samples with 90ul Sample Dilution Buffer. Mix 60ul of the diluted samples with 60ul diluted HRP-RBD solution. Incubate the mixtures at 37°C for 30 minutes. Add 100 µL of the sample mixture to the corresponding wells and incubate at 37°C for 15 minutes. After wash 4 times, add 100 µL of TMB Solution to each well and incubate the plate in dark at 20-25°C for 15 minutes. Then add 50 µL of Stop Solution and read at 450 nm immediately. More than 30% inhibition was determined as neutralizing antibody positive.
Results
S-IgG neutralization assay, cPass sVNT Assay and Cytopathic Effect assay (CPE) result
In the 59 samples from vaccinators, 50 were tested positive by S-IgG assay, and the positive rate is 84.7%. In the 46 health control samples, zero was test positive.
In the 59 samples from vaccinators, 43 were tested positive by cPass sVNT Assay, positive rate is 72.9%. In the 34 samples from no-vaccine people, zero was test positive. As in Figure 1.
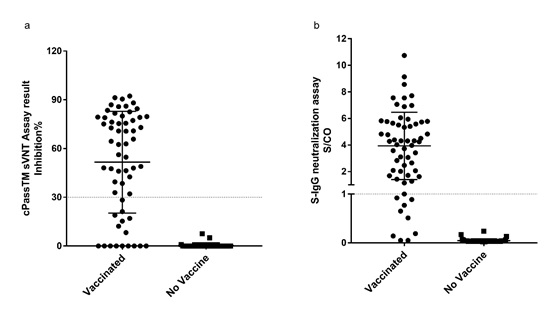
Figure 1: S-IgG neutralization assay and cPassTM sVNT Assay results.
1a: In the 59 samples of vaccinators, 50 were tested positive by S-IgG assay, positive rate is 84.7%. In the 46 health control samples, zero was test positive. 1b: About 43 samples of vaccinators were test positive by cPassTM sVNT Assay, positive rate was 72.9%. In the 34 health control samples, zero was test positive.
The SARS-CoV-2 virus was seeded and propagated in VERO E6 cells. The highest serum dilution that completely protected the cells from CPE in 50% of the wells was taken as the neutralizing antibody titer, and 50% protection was named as SARS-CoV2-CPE++ in Figure 2.
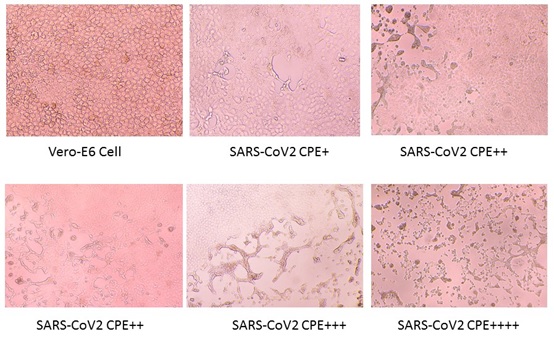 Figure 2: Neutralization experiment, SAS-COV2 virus infects Vero-E6 cells. There are four levels of Cytopathic effect (CPE). A confluent monolayer of Vero E6 cells was prepared in 96 well plate. Serial four-fold dilutions of heat-inactivated sera were made. The serum dilutions 240ul were mixed with equal volumes of 100 TCID50 of SARS-CoV-2 virus. After 2 h of incubation at 37°C in 5%CO2 incubator, 100μL of the virus–serum mixture was added in quadruplicate to Vero E6 cell monolayers in 96-well microtiter plates. Then the plates were incubated 7 days at 37°C in 5% CO2 in a humidified incubator. The CPE was read at 5-days post infection. The highest serum dilution that completely protected the cells from CPE in half of the wells was taken as the neutralizing antibody titer. More than 4 times dilution could protect the cells from virus infection was set as the cutoff of the positive. Below are 4 different levels of the virus infection.
Figure 2: Neutralization experiment, SAS-COV2 virus infects Vero-E6 cells. There are four levels of Cytopathic effect (CPE). A confluent monolayer of Vero E6 cells was prepared in 96 well plate. Serial four-fold dilutions of heat-inactivated sera were made. The serum dilutions 240ul were mixed with equal volumes of 100 TCID50 of SARS-CoV-2 virus. After 2 h of incubation at 37°C in 5%CO2 incubator, 100μL of the virus–serum mixture was added in quadruplicate to Vero E6 cell monolayers in 96-well microtiter plates. Then the plates were incubated 7 days at 37°C in 5% CO2 in a humidified incubator. The CPE was read at 5-days post infection. The highest serum dilution that completely protected the cells from CPE in half of the wells was taken as the neutralizing antibody titer. More than 4 times dilution could protect the cells from virus infection was set as the cutoff of the positive. Below are 4 different levels of the virus infection.
In the 57 vaccinated sample, 47 were tested positive in CPE Assay, positive rate is 82.5%. In the 46 health control samples, zero was test positive. As in Figure 1.
S-IgG neutralization assay and cPass sVNT Assay compared with Cytopathic Effect assay (CPE).
Total 57 vaccinated samples and 5 no-vaccinated samples were compared between S-IgG and CPE. About 43 vaccinated samples both tested positive. Except the 5 no-vaccinated samples, about five vaccinated sample were tested negative in both assays. There are 5 vaccinated samples tested as CPE-/S-IgG+ and 4 vaccinated samples tested as CPE+/S-IgG-. The total positive coincidence rate was 91.5% and the total coincidence rate was 85.5%. As in Table 1.
|
|
S-IgG assay |
cPass sVNT Assay |
|||||
|
+ |
- |
Total |
+ |
- |
Total |
||
|
CPE assay (SARS-CoV2) |
+ |
43 |
4 |
47 |
40 |
7 |
47 |
|
- |
5 |
10 |
15 |
1 |
14 |
15 |
|
|
Total |
48 |
14 |
62 |
41 |
21 |
62 |
|
Table 1: S-IgG neutralization assay and cPass sVNT Assay compared with Cytopathic Effect assay (CPE): Total 57 vaccinated samples and 5 no-vaccinated samples were tested.
Compare S-IgG assay with CPE assay (SARS-CoV2), 43 vaccinated samples both tested positive. Except the 5 no-vaccinated samples, about five vaccinated sample were tested negative in both assays. There are 5 vaccinated samples tested as CPE-/S-IgG+ and 4 vaccinated samples tested as CPE+/S-IgG-. The total positive coincidence rate was 91.5% and the total coincidence rate was 85.5%. As in Table 1.
Compare cPass sVNT Assay with CPE assay (SARS-CoV2), 40 vaccinated samples both tested positive. Except the 5 no-vaccinated samples, about 9 vaccinated sample were tested negative in both assays. There are 1 vaccinated sample tested as CPE-/cPass+ and 7 vaccinated samples tested as CPE+/ cPass-. The total positive coincidence rate was 85.1% and the total coincidence rate was 87.1%. As in Table 1.
Total 57 vaccinated samples and 5 no-vaccinated samples were compared between cPass sVNT Assay and CPE. About 40 vaccinated samples both tested positive. Except the 5 no-vaccinated samples, about 9 vaccinated sample were tested negative in both assays. There are 1 vaccinated sample tested as CPE-/cPass+ and 7 vaccinated samples tested as CPE+/ cPass-. The total positive coincidence rate was 85.1% and the total coincidence rate was 87.1%. As in Table 1.
S-IgG neutralization assay compared with cPass sVNT Assay
Total 59 vaccinated samples and 33 no vaccinated samples were compared. About 43 vaccinated samples were tested positive in both assays. All 33 no vaccinated samples were tested negative in both assays. About 9 vaccinated samples were tested negative in both assays. About 7 more samples were tested positive in S-IgG assay.
The coincidence rate of the positive samples was 100% and the coincidence rate of the negative samples was 85.7%. As in Table 2.
|
S-IgG assay |
cPass sVNT Assay |
Total |
|
|
+ |
- |
||
|
+ |
43 |
7 |
50 |
|
- |
0 |
42 |
42 |
|
Total |
43 |
49 |
92 |
Table 2: S-IgG assay and cPass sVNT Assay comparison: Total 59 vaccinated samples and 33 no vaccinated samples were compared. About 43 vaccinated samples were tested positive in both assays. All 33 no vaccinated samples were tested negative in both assays. About 9 vaccinated samples were tested negative in both assays. There are 7 more samples were tested positive in S-IgG assay.
The coincidence rate of the positive samples was 100% and the coincidence rate of the negative samples was 85.7%. As in Table 2.
Correlation of S-IgG vs CPE assay, cPass sVNT Assay vs CPE assay, and S-IgG vs cPass sVNT Assay
S-IgG assay and CPE were relevant. P=0.0010. cPass sVNT and CPE were relevant too. P

Figure 3: Correlation of S-IgG vs CPE assay, and cPassTM sVNT Neutralization Assay vs CPE assay; and S-IgG neutralization assay vs cPassTM sVNT Neutralization Assay.
S-IgG assay and CPE were relevant. P=0.0010. cPassTM sVNT and CPE were relevant too. P < 0.0001. The linear fitting equation of S-IgG neutralization assay and cPass sVNT Assay results is Y=0.06541*X+0.6056, R squared is 0.6486, Pearson r is 0.8061, and the correlation significance P (two-tailed) < 0.0001. It indicates that these two methods are relevant. All three assays had a good overall correlation.
S-IgG signal increased while sample concentration increased
In the cPass negative and S-IgG tested negative samples (total 16), once increased the sample concentration from 1:100 to 1:50, 1:20 and 1:10 that used in the assay, the ELISA signal increased correspondently. Total 94.9% (56/59) of vaccinators have produced neutralizing antibodies. As in Figure 4.
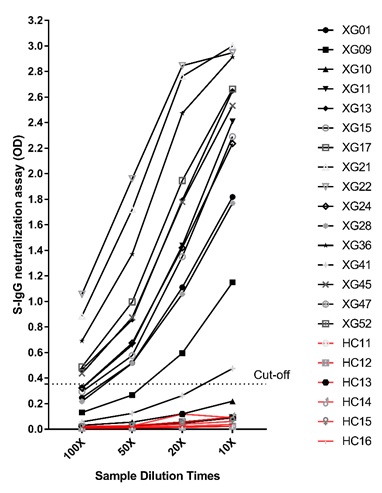
Figure 4: S-IgG at different sample dilution.
In the cPass negative and S-IgG tested negative samples (total 16), once increased the sample concentration from 1:100 to 50, 20 and 10 that used in the assay, the ELISA signal increased correspondently.
Discussion
Although RBD of Spike protein is the primary target of neutralizing antibodies, antibodies that target other antigen motif of S protein may also have neutralization activity. Consisting of S1 receptor-binding subunit and S2 fusion subunit, the SARS-CoV-2 spike (S) protein mediates cell receptor ACE2 binding and cell entry and is the primary target of neutralizing antibodies [15]. The S1 subunit consists of the N-terminal domain (NTD) and the receptor binding domain (RBD). The NTD of the MERS-CoV S protein can serve as a critical epitope for neutralizing antibodies [16]. Depletion of RBD-specific Abs from 21 plasma samples reduced SARS-CoV-2 neutralizing titers by 90% on average [17]. In one report, most of the isolated mAbs did not recognize the RBD, and all the mAbs that neutralize authentic SARS-CoV-2 failed to inhibit the binding of S protein to ACE2 [18]. The S1-targeting mAb 4A8 does not block the interaction between ACE2 and S protein but exhibits high levels of neutralization against both authentic and pseudotyped SARS-CoV-2 in vitro [18]. These unexpected results suggest the presence of other important mechanisms for SARS-CoV-2 neutralization in addition to suppressing the viral interaction with the receptor [18].
Consistent with these discoveries, S-IgG assay was reported to have a high sensitivity in neutralization antibody detection, and a good surrogate of virus neutralization assay [14]. In this report, S-IgG also showed the highest neutralization antibody detection rate as 94.9%.
The cPass™ sVNT Kit was developed that based on the specific protein binding inhibition between the virus surface protein (RBD domain of spike protein) and the human receptor protein (ACE-2). Using 10 times more samples, the cPass sVNT assay detected a positive rate as 72.9%, which is lower than the positive rate as 82.5% of the CPE assay and the 84.2% of the S-IgG assay, as in Table 3.
|
Sample |
Total number |
S-IgG neutralization assay |
cPass sVNT Assay |
Cytopathic Effect assay (CPE) |
|||
|
Positive |
Positive rate |
Positive |
Positive rate |
Positive |
Positive rate |
||
|
Vaccinated |
59 |
50 |
84.7% |
43 |
72.9% |
47 |
82.5% |
|
Health |
46 |
0 |
0.0% |
0 |
0.0% |
0 |
0.0% |
Table 3: S-IgG assay, cPass sVNT Assay and Cytopathic Effect assay (CPE) results.
In the 59 samples from vaccinators, 50 were tested positive by S-IgG assay, and the positive rate is 84.7%. In the 46 health control samples, zero was test positive.
In the 59 samples from vaccinators, 43 were tested positive by cPass sVNT Assay, positive rate is 72.9%. In the 34 samples from no-vaccine people, zero was test positive.
In the 57 vaccinated sample, 47 were tested positive in CPE Assay, positive rate is 82.5%. In the 46 health control samples, zero was test positive. As in Table 3.
To confirm the methods efficiency, we compared the S-IgG assay and cPass-sVNT assay with the tradition virus neutralization Cytopathic Effect assay (CPE). Both methods showed high degree correlations with the Cytopathic Effect assay (CPE) (Figure 3). S-IgG has higher positive coincidence rate (91.5% vs 85.1%) and cPass-sVNA has higher total coincidence rate (87.1% vs 85.5%) with the CPE assay, as in Table 1. Both methods could be used to surrogate the traditional CPE method.
Result also showed that not all of the S protein-binding antibodies were neutralization antibodies, about 5 vaccinated samples were tested as CPE-/S-IgG+. Some neutralization activities were not detected by S-IgG assay, about 4 vaccinated samples were tested as CPE+/S-IgG-, which indicated that the recombinant expressed S protein could not represent all the structure and functions as it on the surface of the virus envelop. Compared with cPass-sVNA assay, about 7 vaccinated samples were tested as CPE+/cPass-sVNA -, which further indicated that the RBD domain of the S protein represented even fewer structure and functions than the S protein on the SARS-CoV2 virus.
Understanding the kinetic and durability of protection from antibody-mediated immune response to SARS-CoV-2 is crucial to understand the pathogenesis of COVID-19, reinfection potential, and vaccine efficacy and development [19]. More follow up experiments will be done to answer the questions such as how long the vaccine induced neutralization antibodies will last? Will inactive vaccine provide more protection than the mRNA vaccine in the long term?
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by grants from:
The leading talents of Guangdong Province program (No.00201512);
National Science and Technology, Major Project (2018zx10732401).
The Key Research and Development Program of Guangdong Province (2019B111103001);
Natural Science Foundation of Guangdong Province (2019A1515012121);
References
- Belete TM (2021) Review on Up-to-Date Status of Candidate Vaccines for COVID-19 Disease. Infect Drug Resist 14: 151-161.
- Hernandez CR, Moreno LS (2020) Immunity against SARS-CoV-2: walking to the vaccination. Rev Esp Quimioter 33: 392-398.
- Singh K, Mehta S (2016) The clinical development process for a novel preventive vaccine: An overview. J Postgrad Med 62: 4-11.
- Cyranoski D (2020) Arab nations first to approve Chinese COVID vaccine despite lack of public data. Nature 588: 548.
- Lipsitch M, Jha A, Simonsen L (2016) Observational studies and the difficult quest for causality: Lessons from vaccine effectiveness and impact studies. Int J Epidemiol 45: 2060-2074.
- Ali MG, Zhang Z, Gao Q, Pan M, Rowan EG, et al. (2020) Recent advances in therapeutic applications of neutralizing antibodies for virus infections: An overview. Immunol Res 68: 325-339.
- Manenti A, Maggetti M, Casa E, Martinuzzi E, Torelli A, et al. (2020) Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol 92: 2096-2104.
- Meyer B, Reimerink J, Torriani J, Brouwer F, Godeke GJ, et al. (2020) Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg Microbes Infect 9: 2394-2403.
- Perera R, Ko R, Tsang OTY, Hui DSC, Kwan MYM, et al. (2021) Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J Clin Microbiol 59: e02504-20.
- Younes S, Al-Jighefee H, Shurrab F, Al-Sadeq DW, Younes N, et al. (2021) Diagnostic Efficiency of Three Fully Automated Serology Assays and Their Correlation with a Novel Surrogate Virus Neutralization Test in Symptomatic and Asymptomatic SARS-COV-2 Individuals. Microorganisms 9: 245.
- Focosi D, Maggi F, Mazzetti P, Pistello M (2021) Viral infection neutralization tests: A focus on severe acute respiratory syndrome-coronavirus-2 with implications for convalescent plasma therapy. Rev Med Virol 31: e2170.
- Tan CW, Chia WN, Qin X, Liu P, Chen MIC, et al. (2020) A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38: 1073-1078.
- Valcourt EJ, Manguiat K, Robinson A, Chen JC, Dimitrova K, et al. (2021) Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn Microbiol Infect Dis 99: 115294.
- Liang L, Huang H, Zheng H, Zhang H, Zhou H, et al. (2020) S-IgG Assay is a Good Replacement of the High Risk, Time Costly Virus Neutralization Assay. J Clin Immunol Immunother 6: 042.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, et al. (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117: 11727-11734.
- Zhou H, Chen Y, Zhnag S, Niu P, Qin K, et al. (2019) Structural definition of a neutralization epitope on the N-terminal domain of MERS-CoV spike glycoprotein. Nat Commun 10: 3068.
- Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, et al. (2020) Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 183: 1024-1042 e21.
- Chi X, Yan R, Zhang J, Zhnag G, ZHnag Y, et al. (2020) A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 369: 650-655.
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, et al. (2020) Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370: 1227-1230.
Citation: Ru Z, Xhang Y, Wu J, Huang H, Liang Y, et al. (2021) Comparison of the SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Assay and Direct Binding ELISA (S-IgG) with the Cytopathic Effect Assay (CPE) in Analyzing the Neutralization Antibody of Vaccination People J Clin Immunol Immunother 7: 063.
Copyright: © 2021 Zhiwei Ru, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

