
Could Pneumoconiosis be an Independent Risk Factor for Pulmonary Embolism? New Knowledge in Old Disease
*Corresponding Author(s):
Bilge AkgunduzDepartment Of Occupational Diseases, Health Science University, Ataturk Chest Diseases And Thoracic Surgery Training And Education Hospital, Ankara, Turkey
Email:bilgeuzmezoglu@hotmail.com
Abstract
Background:The local and systemic cellular response to inorganic dust accumulating in pulmonary parenchyma is still unclear. While TNF-α, IL-6 and fibronectin release due to the dust exposure, cause epithelium cell damage, trigger fibrosis, develop the vascular endothelial dysfunction and activate hypercoagubility, and it is resulted with thrombus formation. In our study, we aimed to determine whether pneumoconiosis plays a role in developing pulmonary thromboembolism (PTE).
Materials and methods:The universe of our study was 2136 patients. The number of patients diagnosed with pneumoconiosis was 539. Patients separated into two groups. Group 1 was defined as patients who diagnosed with PTE with pneumoconiosis. Group 2 was defined as patients who diagnosed with PTE without pneumoconiosis.
Results:We determined 126 (3.12%) patients diagnosed with PTE. 15 (2.78%) patients who had pneumoconiosis were diagnosed with PTE. There was no difference between the mean age of groups (Group 1; n: 54,20 ± 11,38, Group 2; n: 63,87 ± 16,71, p: 0,148). There was no difference between two groups for D-dimer level and diagnostic method. In total, 17 (13,49%) patients had deep vein thrombosis. Silica dust was the dominant exposure agent, and 8 (53,3%) patients had progressive massive fibrosis in Group 2. There was no correlation between Charson Comorbidity Index and PTE.
Conclusion:In our study, presence of PTE in patients with pneumoconiosis suggests that pneumoconiosis has an effect on enhancing coagulation on vascular system.
Keywords
Dust exposure; Pulmonary embolism; Pneumoconiosis
Introduction
Pneumoconiosis is an occupational interstitial lung disease that causes fibrosis in lung parenchyma secondary to inhalation of inorganic dust[1].The local and systemic inflammatory response to dust is unclear[2].Tumor necrosis factor alpha (TNF-α) is the best known cytokine that have been charged with lung fibrosis. While dust triggers to activate alveolar macrophages, also interleukin-1 (IL-1), platelet-derived growth factor (PDGF), transforming growth factor (TGF) and interleukin-6 (IL-6) released during the process of epithelium damage, and these cytokines play role in epithelium damage and systemic inflammation[3].These cytokines also contribute to vascular damage and thrombus formation[4].TNF-α suppresses anticoagulation and fibrinolysis. IL-6 is one of proinflammatory cytokine that activate the coagulation process[5].While reticulin fibers increase with fibroblastic activity, it induces fibrogenic activity. Reticulin deposition has also been found to be associated with ischemic necrosis depending on the vascular injury[6].
It is unknown exactly whether pneumoconiosis is a risk for pulmonary thromboembolism. In a study, D-dimer level was found to be high in patients with pneumoconiosis, and it was found that associated with PTE. It was reported that pneumoconiosis has been found to increase PTE 1.9 fold[7, 8].Tissue damage due to OH- ion on silica surface can trigger vascular endothelial dysfunction and hypercoagulation, and released cytokines may increase hypercoagulation[6, 9].
In this study, we aimed to investigate whether there is an associated between pneumoconiosis and PTE.
Materials And Methods
Data collection:
The study was designed as a retrospective case-control study between January 2014 and December 2019 at Atatürk Chest Diseases and Thoracic Surgery Training and Research Hospital, Department of Occupational Diseases. The database was obtained from our hospital database program. Although patient information was incomplete in database program, data was obtained from patient archive file. While searching pulmonary embolism diagnosis, we used ICD codes. Pre-diagnosis, last-diagnosis and final diagnosis were scanned from the database with ICD codes, and patients with PTE were detected. Our institutional review board approved this observational research, and informed consent was obtained from all patients. Keçiören Education and Research Hospital Ethics Committee approval was obtained with the number of decisions/protocols 18 March 2021/718 for the study.
Patients diagnosed with pneumoconiosis are reported to be presented to hospital health committee for notification to the social security institution. Patients diagnosed with pneumoconiosis were reached from the medical board records’ archive. Patients, who were diagnosed with pneumoconiosis out of our hospital and hospitalized for a different reason, were identified from the hospital database with pneumoconiosis ICD codes.
In five-years period, 2136 patients were hospitalized in department of occupational diseases. 126 patients were diagnosed with pulmonary embolism. Patients separated into two groups. Group 1 was defined as patients who diagnosed with PTE and with pneumoconiosis. Group 2 was defined as patients who diagnosed with PTE and without pneumoconiosis. In this date range, 539 patients were diagnosed with pneumoconiosis (Figure 1).
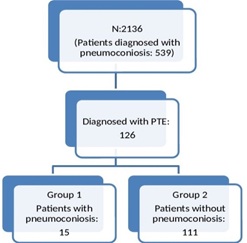
Figure 1: Algorithm of group selection
Charson Comorbidity Index (CCI) score was calculated for each patient. CCI categorized in the two groups, as Score=0 and Score≥1. Patients D-dimer levels were recorded. If C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) analysis were performed at the time of diagnosis, these were also recorded. Patients who had transthoracic echocardiography (ECO) reports were noted.
PTE Diagnosis Criteria:
- Filling defectat the main pulmonary artery and/or segmental, subsegmental branches was determined with Computed Tomography Pulmonary Angiography (CTPA) that reported by a radiologist.
- If there was a contraindication for CT or CT was nondiagnostic, ventilation/perfusion scintigraphy (V/Q SCAN) was performed, and detection of mismatch defects compatible with medium or high probability for pulmonary embolism according to Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) criteria reported by nuclear medicine specialist. Patients diagnosed with pulmonary embolism with CTPA or V/Q SCAN were included in the study.
Statistical analysis:
Variables were analyzed with SPSS-15 version program. Numerical variables that had homogeneous distribution analyzed with t-test and Pearson correlation, and categorical variables analyzed with chi-square test. Non-homogeneous variables analyzed with nonparametric tests.
Results
Characteristics of patients:
126 patients diagnosed with pulmonary thromboembolism were identified. 71 (56.3%) patients were male. The mean age was 62.72 ± 16.43 years. CCI score was 2.95 ± 2.03. COPD was most frequently accompanied. The average DD level of the patients was 1796.64 ± 1763.13 ngr / ml. Diagnostic method was performed with CTPA in 86 (68.3%) patients and with V/Q SCAN in 40 (31.7%) patients. Deep vein thrombosis (DVT) was detected in 17 (13.5%) patients. The number of patients with a genetic risk factor under 40 year-old was within 5 patients. 7 patients had history of surgical intervention. Pulmonary artery pressure (PAP) was 25 mmHg and above in 106 (84.12%) patients (Tables 1 and 2).
|
|
|
Pneumoconiosis, N:15 |
Non-pneumoconiosis, N:111 |
p |
|
|
|
|
|
|
|
Age, year, mean(SD) |
|
54,20±11,38 |
63,87±16,71 |
0,148 |
|
Smoking pack-year, mean(SD) |
|
29,19±18,35 |
31,73±16,58 |
0,925 |
|
Gender n(%) |
Male |
15 (11,9) |
56 (44,4) |
0,000 |
|
Smoking behaviors, n(%) |
Female |
0(0,0) |
55 (43,7) |
|
|
Smoker |
14(12,1) |
46(39,7) |
0,006 |
|
|
Comorbidities, n(%) |
Non-smoker |
1(0,08) |
54(46,6) |
|
|
COPD |
2(1,6) |
18(14,2) |
|
|
|
HT and CAD |
1(0,8) |
21(16,7) |
|
|
|
DM |
1(0,8) |
6(4,8) |
|
|
|
TB |
3(2,4) |
5(3,9) |
|
|
Table 1: Characteristics of patients diagnosed with PTE
There was no statistically significant difference in age, DD level, diagnostic method, and pulmonary arterial pressure among groups (Table 2).
|
|
|
Pneumoconiosis, N:15 |
Non-pneumoconiosis, N:111 |
p |
|
CRP, mg/L, mean±SD |
|
10,46±17,43 |
7,57±18,65 |
0,376 |
|
ERS, mm/saat, mean±SD |
|
32,20±13,66 |
26,62±23,46 |
0,007 |
|
D-dimer, ng/mL, mean±SD |
|
1849,80±1535,02 |
1789,04±1799,81 |
0,616 |
|
PAP, mmHg, mean±SD |
|
42,46±15,70 |
37,43±15,31 |
0,803 |
|
Diagnostic Method, n(%) |
Spiral CT |
11(8,7) |
75(59,5) |
0,450 |
|
V/P Sintigraphy |
4(3,2) |
36(28,6) |
|
|
|
Location of Thrombosis in CTPA, n(%) |
Central |
3(3,5) |
8(9,4) |
0,183 |
|
Segmental/subsegmental |
9(10,6) |
65(76,5) |
|
|
|
PIOPED, n(%) |
Intermediate probability |
3(7,5) |
23(57,5) |
0,562 |
|
High probability |
1(2,5) |
14(35,5) |
|
|
|
CCI Score Category |
CCI Score=0 |
3(2,4) |
17(13,6) |
0,654 |
|
|
CCI Score≥1 |
12(9,6) |
93(74,4) |
|
|
Present DVT, n(%) |
|
0(0) |
17(13,5) |
0,085* |
|
Present Genetic Risk, n(%) |
|
1(0,7) |
5(3,9) |
0,093* |
|
Present surgery history n(%) |
|
2(1,6) |
5(3,9) |
0,479* |
|
Embolism under 40 years old |
|
2(1,6) |
14(12,6) |
- |
|
Present genetic risk factor under 40 years old |
|
0(0,0) |
5(3,9) |
- |
Table 2: Comparison of variables in patients with pneumoconiosis and without pneumoconiosis
Characteristics of patients with pneumoconiosis diagnosed with PTE:
15 patients diagnosed with PTE had pneumoconiosis. PTE point prevalence was found 2.78% in pneumoconiosis patients.
The most common dust was silica. According to the ILO International Classification of Radiographs of pneumoconioses, 8 patients had progressive massive fibrosis (PMF) (Table 3). Six patients with pneumoconiosis did not have comorbidity. In pneumoconiosis group, 12 patients had CCI Score≥1, and 8 of these patients were found exposure to silica dust. There was positive correlation between duration of exposure and CCI score, but this was not statistically significant (correlation coefficient: 0.66; p: 0.07). No relationship was found between exposure agents and comorbidities (p: 0.476). There was no genetic risk factor in patients under 40 years-old with pneumoconiosis that developed PTE. One patient’s PAP was higher than 25 mmHg.
|
Job history |
Values |
|
Duration of dust exposure, year, mean(SD) |
24,40±11,14 |
|
Job, n(%) Sand blasting Stone breaking Welder Digger Dental technician Road construction worker |
4(26,7) 3(0,2) 1(6,7) 2(13,3) 2(13,3) 1(6,7) |
|
Exposure Agent, n(%) Silica Welding fume and metals Silica and metals Coal dust |
10(66,7) 1(6,7) 2(13,3) 2(13,3) |
|
Present PMF, n(%) |
8(53,3) |
Table 3: Characteristics of job history of patients diagnosed with pneumoconiosis
Comparison of CCI scores in two groups:
There was no statistical difference between two groups in regards to the CCI score=0 and the CCI score≥1 (p: 0.654) (Figure 2) Patients whom CCI score was 0, there was no significant difference in DD and CRP levels between the two groups (p: 0.211; p: 0.582, respectively). Patients who had CCI score≥1, there was no significant difference between groups in DD, CRP and PAP levels. The CCI score was higher in without pneumoconiosis group and there was a significant difference between the two groups (1.60 ± 1.18 in the group with pneumoconiosis; 3.13 ± 2.06 in the group with non-pneumoconiosis; p: 0.006).
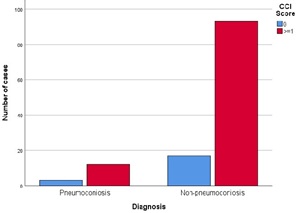 Figure 2: Frequency of CCI score categories in pneumoconiosis and non-pneumoconiosis with PTE
Figure 2: Frequency of CCI score categories in pneumoconiosis and non-pneumoconiosis with PTE
Discussion
According to literature knowledge, our study is the first study that shows, PTE may develop independently from comorbidities in patients diagnosed with pneumoconiosis.
In the large series of patients examined with suspicion of PTE, the prevalence of PTE was reported 10-35%[10].In our study the frequency of PTE was found 6.95%. This frequency doesn’t represent the population, because patients who hospitalized in our department were included in our study. Low prevalence may be related to selection bias. We found the point prevalence of PTE in pneumoconiosis is 2.78%, and we couldn’t find any literature to compare it.
It is unclear whether pneumoconiosis causes pulmonary embolism. In only one study is indicated that pneumoconiosis increases 1,9 fold PTE[8].
Researches have shown that in patients with silicosis and other pneumoconiosis, perivascular fibrosis and muscularization causes damage to the pulmonary arterial structures adjacent to the terminal bronchus which is resulting in vascular stasis[11].Peripheral vein hypoxemia also trigger the pathway that is resulted with local thrombosis[12].A study examined on mousses reported that silica exposure increases pulmonary arterial vascularization and also causes endothelial thickening[13].Erasmus Syndrome and silica-related renal failure are well-known examples of pneumoconiosis related systemic disorders. Studies have supported that inorganic dust exposure may related with systemic inflammation, autoimmunity and vascular damage[14-17].The mechanism of vascular damage and hypercoagulability due to the inorganic dust exposure is unknown. Pneumoconiosis is associated with oxidative stress by activation of TNF-α. While a dust trigger to activate alveolar macrophages, not only TNF-α is secreted, but also IL-1, PDGF, TGF and IL-6are released during the process of epithelium damage. These cytokines have been playing role in vascular damage and coagulation[3, 18].In the light of these hypotheses; our study suggests that pneumoconiosis provides the basis for the development of thrombosis secondary to vascular dysfunction.
COPD is one of the facilitator comorbidity to increase the risk of PTE, such as cancer and oral contraceptive therapy[19].However, we did not find a correlation between CCI score and in terms of embolism in patients with pneumoconiosis and without pneumoconiosis. Also, there was no permanent genetic risk factor in patients with pneumoconiosis. These findings suggest that pneumoconiosis may have a independent risk for developing PTE alone.
Although DVT is presented in non-pneumoconiosis patients, the absence of DVT in patients with pneumoconiosis is supported the hypothesis of pulmonary embolism originates de novo (DNPE), and it is thought that the mechanism of endothelial damage and vascular stasis due to the systemic inflammatory effect in pneumoconiosis[20, 21].
There are some evidences that the silica particles especially crystalline and nanoparticle silica forms pass into systemic circulation with lymphatic system or pass directly through to the vascular area with local endothelial damage [22, 23, 17]. Exposure to ultrafine particles have been shown to increase cardiovascular diseases and DVT.[24, 25]In our study the most common exposure was silica dust and 5 patients were exposed to the freshly shattered silica particles. There are publications that freshly shattered silica has high piozeeletricity and it increases the inflammatory activity and endothelial dysfunction[26].According to the literature knowledge, our study is the first study to investigate the effect of exposure agents on developing PTE, unlike the study conducted by Chen et al[8].
Conclusion
Funding
None.
Conflicts of interest/Competing interests
Authors have not conflict of interest.
Research involving human participants and/or animals
Retrospective Study Involving human participants.
Institution approval
Yes. Health Science University, Atatürk Chest Diseases and Thoracic Surgery Training and Education Hospital; Date-Number: 18.03.2021-718.
Consent to participate
Yes. Our institutional review board approved this observational research, and informed consent was obtained from all patients.
Main Points
- The local and systemic inflammatory response to dust is unclear.
- Exposure to the inorganic dust can trigger to local pulmonary vascular damage, and it can lead to release systemic cytokines which are triggered the coagulation cascade and resulted with thrombosis.
- PTE may develop independently from comorbidities in patients diagnosed with pneumoconiosis
References
- Kefeli M, Akpolat I, Zeren H, Atici AG, Dumortier P, et al. (2012) Clinical, histopathological and mineralogical analysis findings of an unusual case of pneumoconiosis,” Turk Patoloji Derg/Turkish J. Pathol 28:184-8.
- Pollard K M (2016) Silica, silicosis, and autoimmunity. Front Immunol. 11:7:97.
- Vanhee D, Gosset P, Boitelle A, Wallaert B, Tonnel AB (1995) Cytokines and cytokine network in silicosis and coal workers’ pneumoconiosis. Eur Respir J. 8:834-42.
- Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, et al. (2009) Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Respir. J, 34:662-8.
- Davidson SJ (2013) Inflammation and acute phase proteins in haemostasis. In: Janciauskiene S, editor. Acute Phase Proteins. Ch. 2. InTech Publisher; 31.
- Darby IA, Hewitson TD (2007) “Fibroblast Differentiation in Wound Healing and Fibrosis,” International Review of Cytology. 257:143-79.
- Shen CH, Lin TY, Huang WY, Chen HJ, Kao CH, et al. (2015) Pneumoconiosis increases the risk of peripheral arterial disease: a nationwide population-based study. Medicine (Baltimore).94:911.
- Shen CH, Chen HJ, Lin TY, Huang WY, Li TC, et al. (2015) Association between pneumoconiosis and pulmonary emboli. Thromb Haemost 113: 952-957.
- Bauer AT, Strozyk EA, Gorzelanny C, Westerhausen C, Desch A, et al. (2011) “Cytotoxicity of Silica Nanoparticles Through Exocytosis of Von Willebrand Factor and Necrotic Cell Death in Primary Human Endothelial Cells.” 32:8385-93.
- Arseven O, Sevinç C, Alatas F, Ekim N, Erkan L, et al. (2009) “Pulmoner Tromboembolizm Tani VeTedavi Uzlast Raporu (Pulmonary Thromboembolism Diagnosis and Treatment ? Consensus Report). In: Turkish. S. Umut, S.B. Saysal Editors. Introduction. Turkish Thoracic Journal. Aves Publisher; 7-8.
- Hu SU, Vallyathan V, Green FHY, Weber CK, Laqueur W, et al. (1990) “Pulmonary arteriolar muscularization in coal workers’ pneumoconiosis and its correlation with right ventricular hypertrophy,” Arch Pathol Lab Med.114:1063-70.
- Murray J, Reid G, Kielkowski D, De Beer M (1993) “Cor pulmonale and silicosis: A necropsy based case-control study,” British Journal of Industrial Medicine 50:544-548.
- Zelko IN, Zhu J, Ritzenthaler JD, Roman J (2016) “Pulmonary hypertension and vascular remodeling in mice exposed to crystalline silica,” Respir Res. 17:160.
- Zöller B, Li X, Sundquist J, Sundquist K (2012) “Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2:171-83.
- Schreiber J, Koschel D, Kekow J, Waldburg N, Goette A, et al. (2010) Rheumatoid pneumoconiosis (Caplan’s syndrome). Eur J Intern Med. 21:168-72.
- Vupputuri S, Parks CG, Nylander-French LA, Owen-Smith A, Hogan SL, et al. (2012) Occupational silica exposure and chronic kidney disease. Ren Fail. 34:40-6.
- Uzmezoglu B, Simsek C, Gulgosteren S, Gebesoglu BE (2017) Does dust-associated pulmonary alveolar proteinosis represent an autoimmune disorder? Am J Ind Med. 60:591-597.
- Lee JS, Shin JH, Lee JO, Lee WJ, Hwang JH, et al. (2009), “Blood levels of IL-iβ, IL-6, IL-8, TNF-α, and MCP-1 in pneumoconiosis patients exposed to inorganic dusts,” Toxicol Res. 25:217-224.
- Bertoletti L (2017) The paradoxical association between pulmonary embolism and COPD. Eur Respir J. 50:1700959.
- Van Gent JM, Zander AL, Olson EJ, Shackford SR, Dunne CE, et al. (2014) “Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis?J Trauma Acute Care Surg.76:1270-4.
- Roumen-Klappe EM, Heijer MD, Uum SHMV, Ven-Jongekrijg JVD, Graaf FDVD, et al. (2002) “Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 35:701-6.
- Ilinskaya AN, Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part II: Safety concernsNanomedicine (Lond). 8:969-81.
- Dapiaggi M, Pagliari L, Pavese A, Sciascia L, Merli M, et al. (2015) The formation of silica high temperature polymorphs from quartz: Influence of grain size and mineralising agents,” Eur. Ceram. Soc, 235:4447-4555.
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, et al. (2006) Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 173:432-41.
- Baccarelli A, Martinelli I, Zanobetti A, Grillo P, Hou LF, et al. (2008) Exposure to particulate air pollution and risk of deep vein thrombosis,” Arch Intern Med. 168:920-7.
- Jacob J, More N, Kalia K, Kapusetti G (2018) Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen. 38:2.
Citation: Simsek C, Akgündüz B, Sari G (2021) Could Pneumoconiosis be an Independent Risk Factor for Pulmonary Embolism? New Knowledge in Old Disease. J Pulm Med Respir Res 7: 068.
Copyright: © 2021 Cebrail Simsek, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

