
Diurnal Gain and Nocturnal Reduction of Body Weight in Young Adult Rabbits: The Reverse of the Circadian Rhythm Observed in Rats and Mice
*Corresponding Author(s):
Satoshi KawamuraEnvironmental Health Science Laboratory, Sumitomo Chemical Co., Ltd., Kasugade-naka 3-chome, Konohana-ku, Osaka, Japan
Tel:+81 664665055,
Email:kawamuras2@sc.sumitomo-chem.co.jp
Abstract
Understanding circadian rhythms in experimental animals is important to comprehensively evaluate animal responses to chemical exposure and gain deep insight into the pharmacological and toxicological effects of chemical exposure. Animals may respond differently to chemical exposure at different time points because many bodily functions have daily rhythms. In rats and mice, major experimental animals used in toxicology studies, circadian changes in physiological parameters including body weight, food consumption and hormones have been reported. In rabbits, the other principal experimental animal in teratology, circadian rhythms of behavioural functions such as physical activity and food intake, but not body weight change, have been described.
To better understand fundamental biological characteristics of rabbits, we measured body weight and food consumption of male and female rabbits of two strains in the morning and evening for several days, calculating diurnal and nocturnal body weight changes and food intake per hour during the interval.
Rabbits as well as rats and mice ate more at night than during the day. Nevertheless, rabbits showed diurnal increase and nocturnal decrease of body weight. This is the reverse of the circadian change observed in rats and mice. There was no strain-specific difference in the circadian rhythms in body weight and food consumption in rabbits. Male and female rabbits showed a similar daily rhythm in body weight and food consumption. In conclusion, there was a remarkable species difference in circadian rhythm in body weight between rats and rabbits.
Keywords
Circadian rhythm; Diurnal body weight gain; Nocturnal body weight reduction; Rabbits
INTRODUCTION
Physiological responses to chemical exposure in animals may vary depending on the time of day of the exposure since many functions at molecular, cellular, tissue and organism levels show daily rhythms. Understanding circadian rhythms in experimental animals is important to comprehensively evaluate animal responses to chemical exposure and gain deep insight into the pharmacological and toxicological effects of chemical exposure. Circadian changes in body weight and food consumption have been studied in rats along with other functions including urinary concentration [1], water consumption and spontaneous activity [2], taste preferences and fluid intake [3] and hypothalamic ATP [4] and in mice along with water consumption and spontaneous activity [5]. Daily behavioral, hormonal and neurochemical rhythms were also investigated in rats [6]. In rabbits, a principal experimental animal in teratology, daily rhythms of behavioral functions were investigated such as daily rhythms of locomotor activity, food and water intake, hard feces and urine excretion, hematological parameters, serotonin concentration in the brainstem, content and absorption of volatile fatty acids, visual evoked potential and intraocular pressure [7]. There is little information, however, about circadian rhythm in body weight in rabbits.
Our objective in this study was to determine how body weight changes diurnally and nocturnally relative to food intake and using this information, better understand fundamental biological characteristics of rabbits.
MATERIALS AND METHODS
Animals
Ten male and 48 female Japanese White (JW) and 14 male and 40 female New Zealand White (NZW) rabbits were purchased from Nisseiken Co., Ltd. (Tokyo, Japan) and Kitayama Labes Co., Ltd. (Nagano, Japan), respectively and used at age 5 to 7 months. Twenty male and 46 female Sprague-Dawley (SD) rats were from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) and used at age 9 to 10 weeks. Twenty-five female ICR mice were from Japan SLC, Inc. (Shizuoka, Japan) and used at age 9weeks. The animals were individually housed in hanging wire aluminum cages in rooms where air was exchanged more than 10 times per hour. Temperature and humidity were maintained at 22-26°C and 45-65% for rats and mice and at 20-24°C and 45-65% for rabbits. Artificial light was maintained on a 12-h cycle (light on from 8:00 to 20:00). Pelleted diet and tap water were available ad libitum. JW and NZW rabbits were fed NRT-1 (Nisseiken Co., Ltd.) and LRC4 (Oriental Yeast Co., Ltd., Tokyo, Japan), respectively. SD rats and ICR mice were fed CRF-1 (Oriental Yeast Co., Ltd.).
Experimental design
Procedures involving animals and their care were in compliance with Japanese law and were approved by our company’s Institutional Animal Care and Use Committee.
Body weights and food consumption were measured at 9:00 and 16:00 for five consecutive days. Hourly food consumption in the daytime and the nighttime was calculated by dividing food intake during the daytime (i.e. 9:00 through 16:00) by 7 h and food intake during the nighttime (i.e. 16:00 through 9:00 in the next morning) by 17 h, respectively. Body weight change and hourly food consumption were determined for the daytime and the nighttime periods, respectively. Accordingly, the nighttime included a 5-h light period. A schematic of the time schedule of measurement is shown in figure 1. Animals were observed daily for clinical signs.
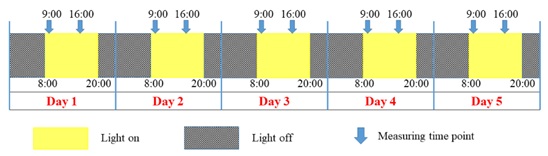 Figure 1: Schematic of the time schedule of body weight and food consumption measurements. The animal rooms were illuminated artificially on a 12-h cycle (light on from 8:00 to 20:00). Body weight and food consumption were measured at 9:00 and 16:00 for five consecutive days.
Figure 1: Schematic of the time schedule of body weight and food consumption measurements. The animal rooms were illuminated artificially on a 12-h cycle (light on from 8:00 to 20:00). Body weight and food consumption were measured at 9:00 and 16:00 for five consecutive days.
RESULTS
Rats and mice
Daily rhythms of body weight and food intake have been reported in rats [1-4] and in mice [5]. In both species, body weight was increased during the night and decreased during the day. Rats and mice ate more in the night time than the daytime. We confirmed these rhythms as shown in figures 2-4. In young female adult rats, body weight changes during the night or the day were much larger than increments of body weight (2-3g) measured every 24h at a fixed point in time such as the peak (9:00) or trough (16:00) time (Figure 2a). Body weight increased by almost 9g, nearly 4% of the body weight, during the night, while the night time increment of body weight was largely lost during the day (Figure 2b). Female rats ate mostly at night and less than 0.2g of the diet per hour during the daytime (Figure 2c). As shown in figure 3, males showed a pattern of daily changes in body weight and food intake similar to that of females. Body weight fluctuation was larger in males than females. Like rats, female mice exhibited an increment of body weight at night and decrement during the day and greater food intake during the night than the day though not to the extent that rats did (Figure 4).
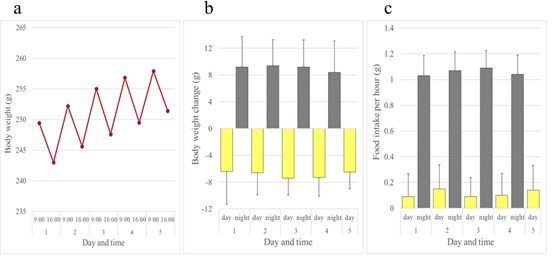 Figure 2: Daily rhythms of body weight and food intake observed in female Crj: CD rats. (a) Body weight over a five-day period. (b) Body weight change over a five-day period. Daytime change in body weight was measured between 9:00 and 16:00. Night time change was measured the following morning between 16:00 and 9:00. (c) Food consumption per hour over a five-day period. Hourly food consumption in daytime and night time was calculated by dividing food intake during the 9:00 through 16:00 period by 7h and during the subsequent 16:00 through 9:00 period by 17 h, respectively. Bars show average + standard deviation.
Figure 2: Daily rhythms of body weight and food intake observed in female Crj: CD rats. (a) Body weight over a five-day period. (b) Body weight change over a five-day period. Daytime change in body weight was measured between 9:00 and 16:00. Night time change was measured the following morning between 16:00 and 9:00. (c) Food consumption per hour over a five-day period. Hourly food consumption in daytime and night time was calculated by dividing food intake during the 9:00 through 16:00 period by 7h and during the subsequent 16:00 through 9:00 period by 17 h, respectively. Bars show average + standard deviation.
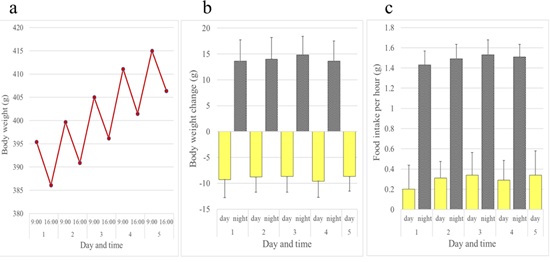 Figure 3: Daily rhythms of body weight and food intake observed in male Crj: CD rats over a five-day observation period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 3: Daily rhythms of body weight and food intake observed in male Crj: CD rats over a five-day observation period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
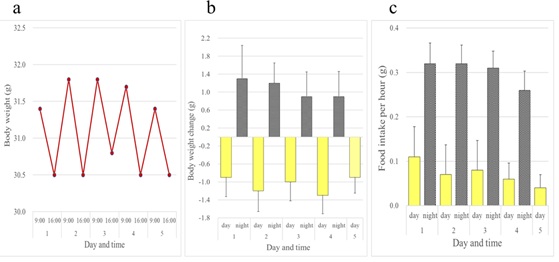 Figure 4: Daily rhythms of body weight and food intake observed in female Slc: ICR mice over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 4: Daily rhythms of body weight and food intake observed in female Slc: ICR mice over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Based on these results, there was no sex-specific difference in circadian rhythms in body weight and food consumption in young adult rats and also, no species difference in these rhythms between female rats and mice.
Rabbits
In contrast to rats and mice, female JW rabbits showed a diurnal increase and nocturnal decrease in body weight. Female rabbits ate all day long, but consumed more of the diet at night than during the day (Figure 5). Although not as evident as females, this inverse pattern of body weight change was also observed in male JW rabbits (Figures 6a and 6b). Food intake was greater at night in males when compared to females (Figure 6c). We investigated whether the daily rhythm of body weight observed in JW rabbits was also found in another strain of rabbits, NZW rabbits. Diurnal gain and nocturnal reduction of body weight were also observed in male and female NZW rabbits. NZW rabbits showed greater food intake at night (Figures 7 and 8).
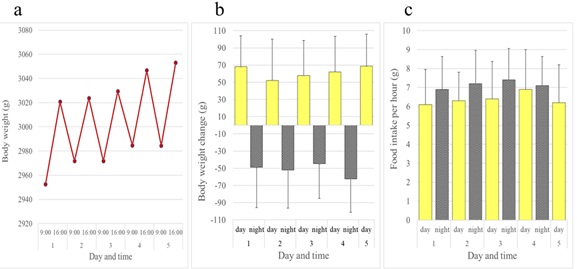 Figure 5: Daily rhythms of body weight and food intake observed in female JW: NIBS rabbits over a five-period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 5: Daily rhythms of body weight and food intake observed in female JW: NIBS rabbits over a five-period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
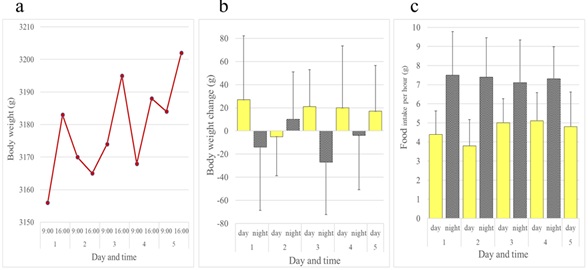 Figure 6: Daily rhythms of body weight and food intake observed in male JW: NIBS rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 6: Daily rhythms of body weight and food intake observed in male JW: NIBS rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
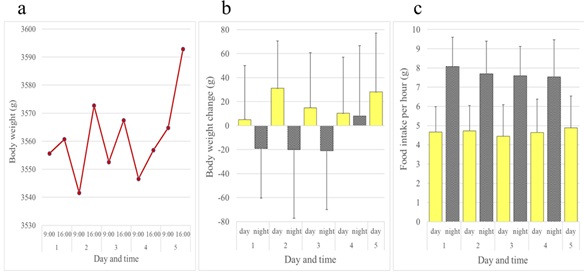 Figure 7: Daily rhythms of body weight and food intake observed in female Kbl: NZW rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 7: Daily rhythms of body weight and food intake observed in female Kbl: NZW rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
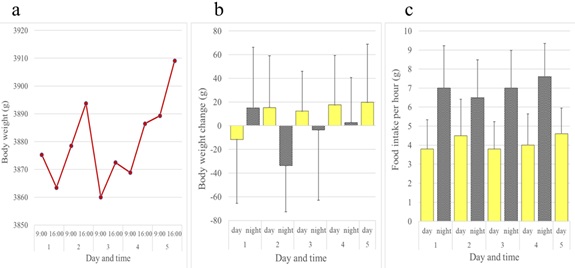 Figure 8: Daily rhythms of body weight and food intake observed in male Kbl: NZW rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
Figure 8: Daily rhythms of body weight and food intake observed in male Kbl: NZW rabbits over a five-day period. (a) Body weights. (b) Daytime and night time body weight changes. (c) Daytime and night time hourly food consumption. Bars show average + standard deviation.
The daytime increment and night time decrement were a small percentage of total body weight. However, as observed in rats and mice, body weight changes during the night or the day were larger than the overall change during a 24h interval. In contrast to rats who eat mostly at night, daytime food intake in rabbits was 60-90% of the night time amount.
In conclusion, there were no strain-specific and sex-specific differences in daily change of body weight and food consumption in rabbits. It is conceivable that, in general, rabbits show diurnal gain and nocturnal loss of body weight, the reverse of the rhythm observed in rats and mice.
DISCUSSION
We conducted this study to investigate rabbit daily rhythms in body weight and food consumption in order to better understand fundamental biological characteristics in rabbits, the principal experimental animals used in teratological studies. In rats, both body weight and food intake increased at night and decreased during the day. The circadian rhythm in food intake in rabbits was similar to that observed in rats. However, the daily rhythm in body weight change in rabbits was the inverse of that observed in rats. While daytime food intake was decreased in rabbits, their daytime body weight was increased.
It is well known that rats are active at night. The rabbit is also innately a nocturnally active animal [8]. The chronological pattern of activity in either animal may be generally similar as shown by the similarity in their nocturnal patterns of food intake. Because the animals used were young adults, they may have been similar in life stage and may have reached a comparable stage of circadian rhythm development. Rabbits attain stable coupling to the light-dark cycle by 45 to 80 days after weaning (at 29 days postpartum), with behavioral functions (locomotor activity, feeding, drinking, micturition and hard feces excretion) occurring in the dark period [9]. In rats, a pattern of nocturnal weight gain reflective of food intake begins at 19 days postpartum, becoming increasingly pronounced thereafter [10]. Gene expression rhythms in the suprachiasmatic nucleus, known as the master clock, are responsive to photoperiod on postnatal day 10 [11]. Expression of Period 1, a core clock gene, in pineal, liver, thyroid, adrenal, and lung tissues peaks during the night by postnatal day 25 [12]. The profile of daily clock genes expression in the adrenal reaches maturity on postnatal day 14 in rats [13]. Consequently, species difference in body weight change would not be due to the pattern of behavioural activity and degree of maturity.
Rats show an estrous cycle as young adults. Individual body weight changes can be influenced by the estrous cycle [14]. Rats were weighed for five consecutive days, during which each rat could be in a state of estrus at least once. Nevertheless, circadian changes of body weight and food intake were comparable from one day to the next during the observation period. The group mean might nullify the impacts of individual estrous cycles. Alternatively, there was no large difference in body weight change among rats in different stages of the estrous cycle in this study. In contrast to ovulation in rats, ovulation in rabbits is initiated by copulatory stimulation and is not cyclical. Regardless of estrous cyclicity, there was no sex-specific difference in circadian change in body weight in either species. This fact suggests that the estrous cycle is not a key factor affecting the difference in daily body weight rhythm between rats and rabbits.
Energy balance is the difference between energy intake and consumption. Energy expenditure is due to the basal metabolic rate, physical activity and diet-induced thermo genesis [15]. In rats, nocturnal gain and diurnal loss of body weight are due to hyperphagia at night and hypophagia during the day [16]. Caloric intake exceeds caloric expenditures at night. During the daytime, caloric intake is lower than the concomitant energy expenditure. The weight gain-weight loss cycle is associated with active lipogenesis during the night time and lipolysis during the daytime. As in previous investigations, this study showed that rats consumed food largely during the night and gained weight at night. While rabbits consumed a considerable amount of food during the daytime, food intake was elevated during the night time when compared to the daytime. This is consistent with a previous report [8]. Rabbits consumed 56% of daily food intake at night. Accordingly, it was unexpected that rabbits gained, rather than lost, weight during the day when they ingested less food than they did at night.
Interestingly, a pattern of behavioural functions observed in normal rabbits is similar to that in hypothalamically-lesioned rats, in which body weight is diurnally increased and nocturnally decreased and daytime and nighttime food intakes are comparable [17]. In addition, mice carrying a mutant Clock gene, a key circadian gene, showed an elevated daytime food intake, consuming nearly 50% of total daily intake, while remaining much less active during the day than at night [18]. The rabbit may be an interesting model to investigate relationships between feeding time, energy balance, body weight control and circadian rhythms.
The present study demonstrated diurnal increment in body weight in the rabbit, which is the reverse observed in the rat. While additional work is required to reach more firm conclusions, we found a remarkable species difference in daily rhythm in body weight between rats and rabbits. To elucidate the basis of this species difference, further investigation will be necessary.
ACKNOWLEDGMENT
The authors would like to thank the staff of Sumitomo’s Developmental and Reproductive Biology Team, especially Mr. Masanao Nakaoka, Mr. Yorihiko Kurata and Mr. Shigenori Kohashi, for their excellent assistance. The authors have no conflicts of interest to declare.
REFERENCES
- Christensen S, Agner T (1982) Effects of lithium on circadian cycles in food and water intake, urinary concentration and body weight in rats. Physiol Behav 28: 635-640.
- Minematsu S, Hiruta M, Watanabe M, Amagaya S (1994) Correlation of body weight gain with food and water consumption and spontaneous activity in rats. Exp Anim 43: 433-438.
- Scalera G (1992) Taste preferences, body weight gain, food and fluid intake in singly or group-housed rats. Physiol Behav 52: 935-943.
- Dworak M, Kim T, McCarley RW, Basheer R (2011) Sleep, brain energy level, and food intake: Relationship between hypothalamic ATP concentrations, food intake and body weight during sleep-wake and sleep deprivation in rats. Somnologie (Berl) 15: 111-117.
- Minematsu S, Hiruta M, Taki M, Fujii Y, Aburada M (1991) Automatic monitoring system for the measurement of body weight, food and water consumption and spontaneous activity of a mouse. J Toxicol Sci 16: 61-73.
- Cuesta M, Clesse D, Pévet P, Challet E (2009) From daily behavior to hormonal an neurotransmitters rhythms: Comparison between diurnal and nocturnal rat species. Horm Behav 55: 338-347.
- Jilge B, Hudson R (2001) Diversity and development of circadian rhythms in the European rabbit. Chronobiol Int 18: 1-26.
- Jilge B (1991) The rabbit: A diurnal or a nocturnal animal? J Exp Anim Sci 34: 170-183.
- Jilge B (1993) The ontogeny of circadian rhyhms in the rabbit. J Biol Rhythms 8: 247-260.
- Levin R, Stern JM (1975) Maternal influences on ontogeny of suckling and feeding rhythms in the rat. J Comp Physiol Psychol 89: 711-721.
- Sumova A, Bendova Z, Sladek M, Kovacikova Z, El-Hennamy R, et al. (2006) The rat circadian clockwork and its photoperiodic entrainment during development. Chronobiol Int 23: 237-243.
- Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, et al. (2009) Ontogeny of circadian organization in the rat. J Biol Rhythms 24: 55-63.
- Roa SLR, Martinez EZ, Martins CS, Antonini SR, Castro M, et al. (2017) Postnatal ontogeny of the circadian expression of the adrenal clock genes and corticosterone rhythm in male rats. Endocrinology 158: 1339-1346.
- Ota K, Yokoyama A (1967) Body weight and food consumption of lactating rats: Effects of ovariectomy and of arrest and resumption of suckling. J Endocr 38: 251-261.
- Johnston JD, Ordovas JM, Scheer FA, Turek FW (2016) Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr 7: 399-406.
- Le Magnen J, Dvos M (1982) Daily body energy balance in rats. Physiol Behav 29: 807-811.
- Kakolewski JW, Deaux E, Christensen J, Carre B (1971) Diurnal patterns in water and food intake and body weight changes in rats with hypothalamic lesions. Am J Physiol 221: 711-718.
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, et al. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043-1045.
Citation: Kawamura S, Yamazoe H, Hosokawa Y (2020) Diurnal Gain and Nocturnal Reduction of Body Weight in Young Adult Rabbits: The Reverse of the Circadian Rhythm Observed in Rats and Mice. J Toxicol Cur Res 4: 016.
Copyright: © 2020 Satoshi Kawamura, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

