
Does Maintenance Therapy for Patients with Substance Use Disorder and Comorbid COVID-19 Infection lead to Adverse Outcomes?
*Corresponding Author(s):
John Arianda Owiti PhD RMNSubstance Misuse Service And Dual Diagnosis Lead, Oxleas NHS Foundation Trust, Forensic And Offender Healthcare Services, HMP Thameside, Griffin Manor Way, London, SE28 0FJ, United Kingdom
Email:john.owiti@nhs.net
Abstract
The safety of Opioid Substitution Therapy (OST) for the treatment of Substance Use Disorder (SUD) in people with Coronavirus Disease 2019 (COVID-19) is not well understood. People with SUD are typically disengaged with healthcare and have multiple comorbidities. Further, opioids are respiratory depressants and could exacerbate respiratory difficulties arising from COVID-19. All of these factors would be expected to put individuals receiving OST at an elevated risk of adverse outcomes during COVID-19.In this retrospective study, we extend the findings from our preliminary publication to evaluate the outcomes of 68 male prisoners detained in His Majesty’s Prison Thameside who contracted COVID-19 while receiving buprenorphine or methadone for SUD. All prisoners with SUD who tested positive for COVID-19 through polymerase chain reaction assay were isolated for 10 days. Blood oxygen levels and pulse readings were monitored daily, and adverse outcomes (overdose, intoxication, sedation, review of medication, and hospital transfer due to deterioration) were recorded throughout the isolation period. Our findings showed that no patients experienced adverse outcomes. These findings concur with those of our preliminary study and, in this patient population, did not show an indication for increased risk of adverse outcomes for OST for patients with SUD who contract COVID-19.
Keywords
COVID-19; Opioid substitution therapy; OST; Prison; Substance use disorder
Introduction
The Coronavirus Disease 2019 (COVID-19)pandemic has led to an increase in Substance Use Disorder (SUD) and disruption to substance use treatment services [1], including those within prisons [2]. Due to the haste with which public health and social restrictions were implemented, adequate risk assessments and care planning to accompany the changes in SUD management were not possible. It was therefore not fully understood whether therapeutic opioids for the treatment of SUD could lead to adverse outcomes for patients with COVID-19.
In prisons, overcrowding, limited access to personal protective equipment, high staff turnover, lack of ventilation in cells, and minimal social distancing make COVID-19 outbreaks likely [3]. Indeed, COVID-19 incidence among prisoners reached three times the level observed in the general population [3]. Furthermore, prisoners often have more complex health needs than the general population [1], including a high rate of SUD. In 2018, 45% of UK prisoners were reported to need treatment for SUD [4], making it crucial to understand the safety of Opioid Substitution Therapy (OST) in people experiencing COVID-19.
Many symptoms of COVID-19 including fever, cough, anosmia and ageusia, shortness of breath, fatigue, loss of appetite, myalgia, sore throat, headache, nasal congestion, runny nose, diarrhea, nausea and vomiting overlap with those of opioid withdrawal [5,6]. This complicates diagnosis and the safe initiation and continuation of OST [6]. Long-term exposure to high-dose opioids, such as in those receiving OST, also affects the respiratory system in ways that could interact with COVID-19 and induce adverse outcomes. Opioids can trigger respiratory depression through activation of μ-opioid receptors in the brainstem, leading to respiratory failure [7,8]. People with COVID-19 are also at elevated risk of silent hypoxia, a severely low level of oxygen in the blood, without the usual signs of shortness of breath [9]. The two effects together may be expected to increase the risk of progression to respiratory failure.
To investigate the scale of this risk in the prison setting, we conducted a preliminary observational study at a male remand prison between January and February 2021, where we recorded adverse outcomes among 23 prisoners with SUD receiving OST who tested positive for COVID-19 [10]. Here, we expand on this study by including a further 45 patients from the same prison to identify any safety issues arising from OST during COVID-19 more robustly.
Methods
This was a retrospective audit of the prison Electronic Medical Record (EMR) system at His Majesty’s Prison (HMP) Thameside, a male remand prison in South London, UK, that receives an average of 90 prisoners a month requiring treatment for SUD. All patients who tested positive for COVID-19 between 28/12/2020 and 27/12/2021, and were prescribed either buprenorphine or methadone for SUD, were monitored for adverse outcomes. Testing, isolation, and monitoring procedures were described in Owiti et al., [10] and are shown in figure 1. Samples for Polymerase Chain Reaction (PCR) testing were collected by nasopharyngeal swab. These were sent to a designated pathology network laboratory run by a local National Health Service (NHS) hospital following NHS protocols [11]. The results were entered into the testing laboratories database which was linked to patients’ records through the EMR system, SystmOne. Prison healthcare staff could check the records immediately. All prisoners who tested positive were isolated for 10 days with any pre-existing cellmate.
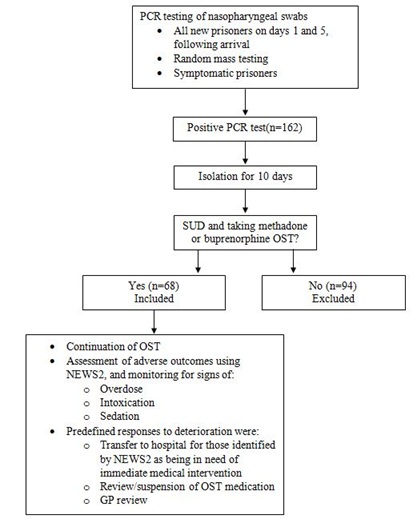 Figure 1: Patient flow.
Figure 1: Patient flow.
COVID-19, Coronavirus Disease 2019; GP, General Practitioner; NEWS2, National Early Warning Score 2;OST, Opioid Substitution Therapy; PCR, Polymerase Chain Reaction; SUD, Substance Use Disorder.
Adverse outcomes were defined as overdose, intoxication, sedation, medication review, and deterioration requiring hospital transfer. Deterioration, including signs and symptoms of adverse outcomes, was monitored using pulse rate and oxygen saturation, with oxygen saturation below 94% (or below 88% in the presence of carbon dioxide-retaining chronic lung disease) and a pulse outside a resting rate of 60-100 beats per minute (bpm) considered outside the ideal range. Patients with pulse rates outside the normal range were re-monitored on the day and daily afterwards during the period of isolation. They could only receive medication if their pulse rate was >60 bpm after re-monitoring. The National Early Warning Score 2 (NEWS2) scoring system was also used to identify patients at risk of deterioration [12]. NEWS2 was used alongside a full clinical assessment, and within the context of the changes over time and existing medical conditions. NEWS2 values were generated automatically, with alerts requiring escalating clinical responses for increased scores (Supplementary Table 1). Prior to medication administration each morning, patients’ symptoms, oxygen saturation, and pulse, together with any signs of overdose, intoxication, and sedation were recorded throughout their 10-day isolation period. Where deterioration was noted, clinical assessment and the NEWS2 were used to identify patients requiring immediate medical intervention.
Results
Between February 2020 and April 2022, 162 prisoners tested positive for COVID-19. Of these, 68 were eligible for inclusion in our analysis. Demographic characteristics of this group are detailed in table 1. Mental health disorders were present in 72%, with 16% having two or more diagnoses. Physical illness was less prevalent, with 17 patients (25%) having a non-psychiatric diagnosis. Methadone was the most prescribed OST, and 44 patients (65%) were receiving a daily dose of ≤40 mg. Alongside OST, the majority (76%) of patients were taking medication for comorbid conditions, with mirtazapine being the most prescribed.
Over one-half (57%) of patients on OST who tested positive for COVID-19 did so within 5 days of arrival. A minority of patients (15%) had symptoms of COVID-19 and, among these, fever was the most common symptom (80%). Oxygen saturation remained ≥92% in all patients throughout their 10-day isolation periods. Six patients experienced pulse rates outside the predefined ideal range, for a period of1or 2days, with rates varying from 55 to 111 bpm. Clinical assessment of these patients, together with NEWS2 screening and normal blood oxygen readings deemed them to be without need for further medical intervention. Signs of overdose, intoxication, or sedation were not observed in any of the patients throughout their 10-day isolation period. Hospital transfer was not required at any time.
|
Median age, years (range) |
38 (20-64) |
|
Ethnicity, n (%) |
|
|
Caucasian |
47 (69) |
|
Black |
11 (16) |
|
Asian |
10 (15) |
|
Diagnosed physical health disorder, n(%) |
18 (26) |
|
Asthma |
7 (10) |
|
Hypertension |
3 (4) |
|
Pain |
3 (4) |
|
Gastroesophageal reflux |
2 (3) |
|
Type 2 diabetes |
2 (3) |
|
Epilepsy |
1 (1) |
|
Hepatitis C |
1 (1) |
|
Human immunodeficiency virus |
1 (1) |
|
Diagnosed mental health disorder, n (%) |
49 (72) |
|
Depression |
33 (49) |
|
Schizophrenia |
16 (24) |
|
Anxiety |
5 (7) |
|
Post-traumatic stress disorder |
4 (6) |
|
OST prescribed prior to prison entry, n (%) |
29 (43) |
|
Reason for testing among COVID-19-positive cases, n (%) |
|
|
Day 1 of prison entry |
11 (16) |
|
Day 5 of prison entry |
28 (41) |
|
Mass testing |
11 (16) |
|
Symptomatic |
7 (10) |
|
Cellmate tested positive |
26 (38) |
|
Buprenorphine ≤4 mg, n (%) |
3 (4) |
|
Buprenorphine >4 mg, n (%) |
16 (24) |
|
Methadone ≤40 mg, n (%) |
44 (65) |
|
Methadone >40 mg, n (%) |
5 (7) |
|
Receiving medications (other than OST), n (%) |
52 (76) |
|
Receiving ≥2 medications, n (%) |
20 (29) |
|
Mirtazapine |
7 (10) |
|
Olanzapine |
6 (9) |
|
Salbutamol |
6 (9) |
|
Sertraline |
5 (7) |
Table 1: Demographic characteristics.
COVID-19, Coronavirus Disease 2019; OST, Opioid Substitution Therapy.
Discussion
Over one-half of patients on OST for SUD who tested positive for COVID-19 did so within 5 days of arrival at the prison. This is a key time for the safe initiation or continuation of OST when signs of intoxication, withdrawal, or sedation are closely monitored. At no time were these adverse outcomes seen, and no medication reviews outside of normal protocol were necessary. However, of the 10 patients who had symptomatic COVID-19 infection, only five tested positive within 5 days of arriving. Therefore, overlapping symptoms between COVID-19 and intoxication, withdrawal, and sedation would have been a factor for a small minority of patients. As in our preliminary study [10] no adverse outcomes were observed at any time during the 10-dayisolation period among prisoners receiving OST who contracted COVID-19. Although some patients had pulse rates outside the ideal range, clinical assessment together with NEWS2 did not indicate the need for escalation.
Comparative data for outcomes among individuals with COVID-19 on OST are lacking. However, a systematic review and meta-analysis [13] showed opioids to have significant associations with both intensive care unit admission and mortality. A previous retrospective case-control study of electronic health records [14] found that hospitalization and death due to COVID-19 infection were more common in patients with SUD than in those without. However, the prescription of OST is not mentioned in this study. A large-scale, retrospective cohort study in the USA [7] used EMR data and propensity score matching to assess the risk of morbidity and mortality from COVID-19 among 9558 adults treated with long-term opioids for chronic non-cancer pain. While only a minority might also have been treated with OST for SUD, patients on long-term opioid treatment were found to be at increased risk of morbidity, mortality, and healthcare utilization during COVID-19 infection. Both these studies report higher rates of symptomatic COVID-19 than that observed within our dataset. In addition to receiving OST, most patients in our study had at least one mental health disorder, and many of these patients were being treated with psychotropic medications. There is some evidence that people with COVID-19 taking psychotropic medications may be at risk of respiratory, cardiovascular, infective, hemostatic, and consciousness alterations [15]. Although this risk was elevated in those also on OST, we did not see these adverse outcomes in our study.
It is unclear whether the characteristics of our prison cohort affected the risk of adverse outcomes. An observational study [16] described the clinical characteristics and outcomes of people with SUD who were admitted to a public hospital for COVID-19. Of the 2078 patients admitted, only 27 people had SUD. This small minority were typically >50 years old, with a high prevalence of comorbidities. In comparison, the median age of patients in our study was 38, with only four patients aged ≥50 years. Although 76% were being prescribed medications for comorbid conditions, few had physical conditions associated with adverse outcomes during COVID-19. Olanzapine and pregabalin, prescribed to two and one patient, respectively, were the only medications associated with a risk of respiratory depression [17,18]. It is therefore possible that the lack of adverse outcomes within our study may be due to a younger and healthier than expected population. Further, most of our study population were on relatively low doses of OST, as they were still on induction into treatment and had not yet reached their therapeutic maintenance dose. The effect of OST dose on adverse outcomes is yet to be established and warrants further investigation.
We believe that high rates of COVID-19 and SUD within prison populations make this research necessary and timely. Despite the expectation that people taking OST for SUD would be at elevated risk of adverse outcomes during COVID-19, our study does not indicate an increased safety risk. Our study does not indicate any need to disrupt OST dosing if a patient contracts COVID-19 in prison. However, further studies are needed and the impact of symptomatic versus asymptomatic COVID-19, and of OST, dose should be considered.
Funding
Medical writing support was funded through the Opi-Assist program (https://www.opi-assist.com/). Opi-Assist is funded through a donation from Camurus. Camurus has no influence on the selection of the steering committee, or the selection of applications awarded medical writing support by the Opi-Assist program.
Disclosures
No potential conflicts of interest were reported by the author(s).
Data Availability
Data are available on reasonable request.
Acknowledgement
The authors thank Meredith Jones, PhD (Alchemie Medical Education) for medical writing support, funded through the Opi Assist program (https://www.opi-assist.com/).
References
- Dubey MJ, Ghosh R, Chatterjee S, Biswas P, Chatterjee S, et al. (2020) COVID-19 and addiction. Diabetes Metab Syndr 14: 817-823.
- Trayner KMA, McAuley A, Palmateer NE, Yeung A, Goldberg DJ, et al. (2022) Examining the impact of the first wave of COVID-19 and associated control measures on interventions to prevent blood-borne viruses among people who inject drugs in Scotland: An interrupted time series study. Drug Alcohol Depend 232: 109263.
- Coleman PC, Pailing A, Roy A, O'Moore É, Chandan JS, et al. (2022) Implementation of novel and conventional outbreak control measures in managing COVID-19 outbreaks in a large UK prison. BMC Public Health 22: 677.
- Ministry of Justice (2021) Prisons strategy white paper. Ministry of Justice, UK.
- UK Health Security Agency (2023) COVID-19: epidemiology, virology and clinical features. UK Health Security Agency, UK.
- Mostafa Mirakbari S (2022) COVID-19 infection masquerading as recurrent apnoea in acute opioid overdose. Arh Hig Rada Toksikol 73: 178.
- Tuan WJ, Spotts H, Zgierska AE, Lennon RP (2021) COVID-19 outcomes among adult patients treated with long-term opioid therapy for chronic non-cancer pain in the USA: A retrospective cohort study. BMJ Open 11: 056436.
- Zibbell J, Howard J, Clarke S, Ferrell A, Karon SL (2019) Non-fatal opioid overdose and associated health outcomes: Final summary report. U.S. Department of Health and Human Services, Washington, D.C., USA.
- Luks AM, Swenson ER (2020) Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc 17: 1040-1046.
- Owiti JA, Benson M, Maplanka M, Oluseye L, Carvalho D (2022) Is methadone safe for patients with opioid use disorder and coronavirus disease 2019 infection? J Addict Nurs 33: 86-94.
- National Health Service England (2021) Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings. National Health Service England, England, UK.
- Royal College of Physicians (2017) National Early Warning Score (NEWS) 2. Royal College of Physicians, London, UK.
- Ao G, Li A, Wang Y, Li J, Tran C, et al. (2022) Opioid usage and COVID-19 prognosis: A systematic review and meta-analysis. Am J Emerg Med 56: 51-56.
- Wang QQ, Kaelber DC, Xu R, Volkow ND (2021) COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry 26: 30-39.
- Ostuzzi G, Papola D, Gastaldon C, Schoretsanitis G, Bertolini F, et al. (2021) Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations. BMC Med 18: 215.
- Vallecillo G, Perelló R, Güerri R, Fonseca F, Torrens M (2020) Clinical impact of COVID-19 on people with substance use disorders. J Public Health (Oxf) 43: 9-12.
- National Institute for Health and Care Excellence (2023) Olanzapine. NICE, London, UK.
- National Institute for Health and Care Excellence (2023) Pregabalin. NICE, London, UK.
Supplementary Table
|
NEWS score |
Frequency of monitoring |
Clinical response |
|
0 |
Minimum 12 hourly |
· Continue routine NEWS2 monitoring |
|
1-4 |
Minimum 4-6 hourly |
· Inform registered nurse, nurse in charge, or Hotel 1*, who must assess the patient · Registered nurse, nurse in charge, or Hotel 1* decides whether increased frequency of monitoring and/or escalation of care is required · Repeat immediately |
|
3 in a single parameter |
Minimum 1 hourly |
· Registered nurse, nurse in charge, or Hotel 1* to inform the general practitioner (GP), who will review and decide whether escalation of care is necessary · Use the situation, background, assessment, recommendation, and decision (SBARD) tool to report, escalate and record deterioration |
|
|
Minimum 1 hourly |
· Registered nurse, nurse in charge, or Hotel 1* to request urgent assessment by a GP or advanced nurse practitioner (ANP) · Provide clinical care in an environment with monitoring facilities · Use SBARD tool to report, escalate, and record deterioration |
|
|
Continuous monitoring of vital signs |
· Registered nurse, nurse incharge, or Hotel 1* to immediately call CODE BLUE and an ambulance · Registered nurse, nurse in charge, or Hotel 1* to escalate immediately to GP, Lead Clinician/Manager · Use SBARD tool to report, escalate, and record deterioration |
*The designated senior nurse that leads the response to emergencies.
ANP, Advanced Nurse Practitioner; GP, General Practitioner; NEWS2, National Early Warning Score 2; SBARD, Situation, Background, Assessment, Recommendation, and Decision.
Citation: Owiti JA, Benson M (2023) Does Maintenance Therapy for Patients with Substance Use Disorder and Comorbid COVID-19 Infection lead to Adverse Outcomes? J Addict Addictv Disord 10: 122.
Copyright: © 2023 John Arianda Owiti PhD RMN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

