
Effect of Different Oxygen Tension on Culture and Growth Pattern of Horse Adipose Tissue and Umbilical Cord Blood Derived Mesenchymal Stem Cells (hrs-AT-MSC and hrs-UCB-MSC)
*Corresponding Author(s):
Swapan Kumar MaitiPrincipal Scientist, Surgery Division, ICAR-Indian Veterinary Research Institute, Izatnagar-243122 UP, India
Email:maiti_62@rediffmail.com ; swapanivri@gmail.com
Abstract
Equine multipotent mesenchymal stem cells (hrs-MSC) can be isolated from various tissues including Adipose Tissue (AT) and Umbilical Cord Blood (UCB). We have analyzed the effect of different oxygen tension on hrs-AT-MSC and hrs-UCB-MSC cultured and assessed proliferation, morphology, viability and immunophenotype and plasticity. Both type of equine MSC were cultured separately in normoxic (21% O2) and hypoxic (5% O2) environment. In hypoxic environment, MSC exhibited a significantly different morphology with shorter extensions and broader cell bodies. MSC did not proliferate as rapidly as under 21% oxygen. Number of cell colonies as well as sizes of cell colonies was much higher in normoxic than hypoxic environment. The immunophenotype with Integrin alpha 5 expressions was higher in normoxic than hypoxic conditions. In conclusion, physiologic oxygen tension (5%) during in vitro culture of equine MSC derived from adipose tissue and umbilical cord blood slows down the cell cycle progression and differentiation. Further studies are needed to analyze these aspects of MSC in tissue regeneration.
Keywords
Adipose tissue; Equine; Mesenchymal stem cell; Oxygen tension Umbilical cord blood
Abbreviations
MSC: Mesenchymal Stem cell
Hrs-MSC: Horse Mesenchymal stem cell
Hrs-AT-MSC: Horse Adipose Tissue derived Mesenchymal Stem Cell
Hrs-UCB-MSC: Horse Umbilical Cord Blood derived Mesenchymal Stem Cell.
EDTA: Ethylene Diamine Tetra Acetic Acid
DMEM: Dulbecco’s Modified Eagle Medium
FBS: Fetal Bovine Serum
PBS: Phosphate Buffered Saline
DPBS: Dulbecco’s Phosphate Buffered Saline
FACS: Fluorescein -Activated Cell Sorting
O2: Oxygen
Introduction
Stem cell biology has attracted tremendous interest recently. It is hoped that it will play a major role in the treatment of a number of incurable diseases via transplantation therapy. Several verities of stem cells have been isolated and identified in vivo and in vitro. Very broadly they comprise of two major classes: embryonic and adult mesenchymal stem cells [1]. Isolation, proliferation and morphological characteristics of bone-marrow derived mesenchymal stem cells from pig, rabbit, dog, sheep and goat has been reported [1]. Isolation of MSC derived from equine species has been reported in a number of different tissues, including bone marrow [2], fat tissue [3], umbilical cord blood [4] and peripheral blood [5]. Equine MSC derived from adipose tissue and umbilical cord blood show the capacity to differentiate under appropriate culture conditions using specific hormones and growth factors in mesodermal lineages, such as bone, cartilage and fat [3,4].
The use of MSC for tissue engineering purposes and their potential to differentiate along the bone, cartilage, fat, and muscle lineages have been demonstrated, and their usefulness for regenerative medicine approaches is clear [6]. MSCs might exert their beneficial effects in various ways. They might differentiate directly and function as a new cell type, but they might also function indirectly through the release of trophic factors that fulfil an endocrine/paracrine role. Their harvest from autologous donor site is relatively harmless and they can readily be cultured in vitro to obtain sufficient cell numbers for re-implantation [7].
The issue of optimal culture conditions for cells has been under investigation for many years. An important consideration is the optimum oxygen tension in which to culture cells. Physiological oxygen tension varies from as much as 12% in the blood to as low as 1% in the deep zone of cartilage regions [8]. The oxygen tension in bone marrow has been described to be between 4% and 7% [9]. In any case, the oxygen tension is considerably lower than the atmospheric oxygen tension (21%) of standard cell culture [10].
Oxygen tension is a crucial parameter for the culture of MSCs. It is an important biochemical signalling molecule for all major aspects of stem cell biology, including self-renewal, proliferation, cell death, differentiation and migration [8,11]. Hypoxia plays a key role in various patho-physiological process and tissue regeneration. Reduced O2 concentration affects MSC viability and proliferation activity, which modulates reparative processes in the tissue. Experiments on MSC culturing under hypoxic conditions showed the necessity of studying various aspect of MSC function in the presence of low O2 concentration [12].
In this study, the effect of normoxic (21%) and hypoxic (5%) oxygen tension on proliferation, viability and phenotypic characteristics of equine MSC derived from adipose tissue (hrs-AT-MSC) and umbilical cord blood (hrs-UCB-MSC) was investigated.
Materials and Methods
Materials used
We have used Dulbecco Modified Eagles Media (DMEM) (Gibco) containing 100U/ml penicillin, 100 mg/ml streptomycin, and 10% FBS (fetal bovine serum) (Gibco), culture flasks and Petri dishes (Nunc) for cell culture, phosphate buffered saline (20mM, Gibco) for cell washing; 0.02% trypsin with 0.05% EDTA (Gibco) for cell harvesting.
Cell viability test
Cell viability was estimated by the method of flow cytofluorometry using Propidium iodine (Pi) stain. Morphological characteristics of MSC were evaluated under an Axiovert 25 phase contrast microscope (Zeiss) connected to a digital camera. Proliferative activity of MSC cultures was determined by cell counting with the help of Casy I cell counter on day 0, 1, 2, 3, 4, 5, 6, 7 and 8 cultures under normoxic or hypoxic conditions. The significance of differences was estimated by nonparametric Mann-Whitney test.
The study was conducted with passages 1-4 of MSC cultures isolated from equine adipose tissue and umbilical cord blood.
MSC isolation
Adipose tissue and umbilical cord blood derived MSCs were isolated from equine donors as previously described [3,4]. For UBC blood, cells were obtained using a commercially available kit, as per the manufacturer’s instructions, followed by Ficoll-Paque density gradient centrifugation, and plated in non-coated tissue culture flasks in expansion medium (Gibco). Cells were allowed to adhere overnight and non-adherent cells were washed out with medium changes. Medium changes were carried twice weekly thereafter.
Limiting dilution
After 7-10 days at near confluence of the cultures, the cells were detached using trypsin/EDTA. The cells were passage at 5x103cells/cm2 and cultured to reach 80-90% confluence at passsage2 (P2).
Maintenance and culture expansion
Once adherent cells reached approximately 80 % to 90% confluence, they were detached with trypsin-EDTA, washed twice with phosphate-buffered saline (PBS), with centrifugation, 1000 rpm for 5 minutes, and re-culture in Co2 incubator for next passaging.
Hypoxia condition
Hypoxia of the culture medium was induced in a sealed chamber (Stem Cell technologies) by delivering a gas mixture of 95% N2 and 5% CO2 until O2 concentration in the medium was brought to 5% O2 content and gas pressure were monitored using chamber sensors. Control cells were maintained in a CO2 incubator under standard normoxic conditions (5% CO2, 21% O2).
Colony-Forming Unit-Fibroblast assay (Immunophototyping)
After isolation of mononuclear cell (P4), 2x104 MSC/cm2 were seeded in 10cm Petri dishes. After 14 days, colonies were washed with PBS and fixed with methanol for 5 minutes. After removal and air-drying (5 min), cultures were incubated for 5 min in Giemsa stain solution. Cultures were washed twice with water and colonies with more than 50 MSCs were counted.
Flow cytometry/ Immunophenotyping
About 3×105 hrs-AT-MSCs and hrs-UCB-MSCs were collected by centrifugation at 500g for 5 min, suspended in 50 μl PBS containing 1% (w/v) human serum albumin, and labelled with the conjugated primary antibodies (Integrin alfa 5 ). The samples were incubated for 30 min at room temperature. The samples were then analyzed by flow cytometry (FC; FACScalibur, BD Biosciences, San Jose, Calif., USA) with CellQuest software. To assay cells by the forward and side scatter of light, the instrument was standardized with microbeads (Dynosphere Uniform Microspheres, Bangs Laboratories, Fisher Scientific, USA).
Cell cycle analysis
Cell cycle analysis of MSC was performed after 7 days and 21 days of adaptation in 21% O2 and 5% O2. 1x106 MSC were washed with phosphate buffered saline three times, fixed with ice-cold 70% ethanol and stored at 4ºC for a minimum of 1 hour. After three more washing steps with PBS, DNA of the MSC was stained with propidium iodide. The samples were incubated at room temperature for 15 minutes and analyzed by flow cytometry on a FACS Caliber (Becton Dickinson, Heidelberg, Germany).
Results
Primary culture of both hrs-AT-MSC and hrs-UCB-MSC consisted of cells of the same type and actively proliferate. However, after sub culturing the population of MSC became heterogeneous and contained several types of cells differing in morphological characteristics and proliferative activity. The culture of both types (AT and UCB) of MSC included the following cells: actively proliferating spindle-shaped or triangular cells with homogenous cytoplasm; actively proliferating and spread fibroblast-like cells with large nucleus; and slowly proliferating and highly spread cells that had a polygonal, oval, or irregular shape and heterogeneous cytoplasm. Proliferative activity in most cultures decreased by 4th passage, slowly dividing and spread cells predominated. Further sub culturing abolished this so-called “proliferative inhibition”, proliferative activity increased and remained at a high level, which led to predominance of morphologically similar cells in the culture.
Morphological characteristics of MSC were studied during culturing under various conditions. Normoxia stimulated the formation of islets from small cells with optically homogenous cytoplasm. These cells were in close contact with each other and formed colonies. The number and size of colonies were higher under normoxic conditions. Normoxia was also accompanied by a decrease in the ratio of large, spread, and slowly dividing cells with different size and various morphological characteristics. Normoxia decreased heterogeneity of MSC cultures and increased the percent of small cells of a similar type. Whereas, in hypoxia condition, cell size and cell colonies and cell proliferative activity were reduced. In hypoxia, MSC exhibited a significant different morphology with shorter extensions and broader cell bodies (Figure 1).
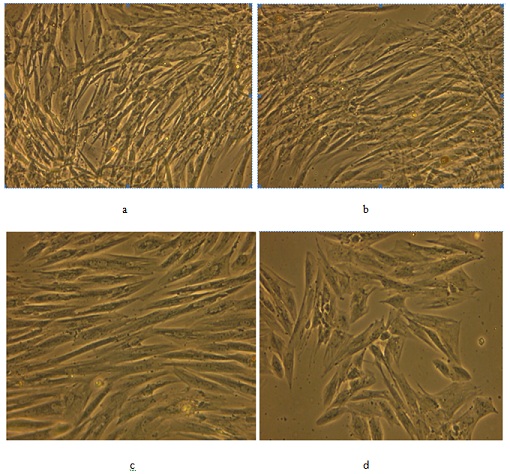 Figure 1: MSC morphology under different O2 tension: Morphology under normoxia conditions in AT (a) and UCB (b) derived hrs- MSC; Morphology under hypoxia conditions in AT (c) and UCB (d) derived hrs- MSC.
Figure 1: MSC morphology under different O2 tension: Morphology under normoxia conditions in AT (a) and UCB (b) derived hrs- MSC; Morphology under hypoxia conditions in AT (c) and UCB (d) derived hrs- MSC.
Cell cycle analysis of hrs-MSC after a period of 7 days at the standard culture conditions revealed that only 1.67% of MSC had entered the G2/M phase in the hypoxic cell culture in comparison to 2.80% at an oxygen concentration of 21%.
MSC did not proliferate in hypoxia condition as rapidly as under 21% oxygen (Figure 2).
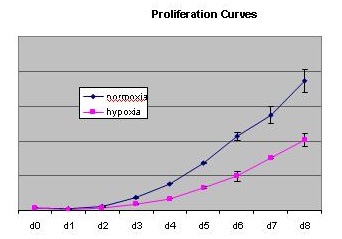 Figure 2: Proliferation of hrs AT-MSC cells under different oxygen tension conditions.
Figure 2: Proliferation of hrs AT-MSC cells under different oxygen tension conditions.
Cell population and of both hrs-AT-MSC and hrs-UCB-MSC were higher in normoxic condition than hypoxia (Table 1).
|
Normoxia |
Hypoxia |
|
• hrs-AT-MSC : 8.11 million/ml • Hrs-UCB-MSC: 3.66 million/ml • Average diameter: hrs-AT-MSC:17.43 µm
|
• hrs-AT- MSC :8.09 million/ml • Hrs-UCB-MSC: 3.59 million/ml • Average diameter: hrs-AT-MSC:16.95 µm
|
Table 1: Cell count and cell diameter.
Viability of hrsMSC under different oxygen tension is depicted in figure 3. The viability of both hrs-AT-MSC and hrs-UCB-MSC was higher in normoxic condition than hypoxic.
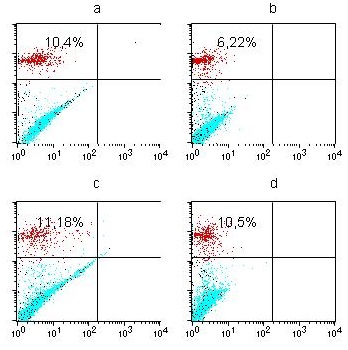 Figure 3: Viability of hrs MSC under different O2 tension: hrs AT-MSC under normoxia (a), hypoxia (b); hrs UCB-MSC under normoxia (c), hypoxia (d).
Figure 3: Viability of hrs MSC under different O2 tension: hrs AT-MSC under normoxia (a), hypoxia (b); hrs UCB-MSC under normoxia (c), hypoxia (d).
After 14 days, cultures that originated from 2x104 mononuclear hrs-AT-MSC (P4) at 5% O2 displayed 24 ± 5 CFU-F while CFU-F numbers at 21% O2 averaged 47 ± 6 and were thus about 2 –fold higher compared to 5% O2 cultures. Beside differences in CFU-F numbers, colonies in high (normoxic) oxygen environment were found to be larger in diameter (Figure 4), in agreement with increased proliferation at 21% O2.
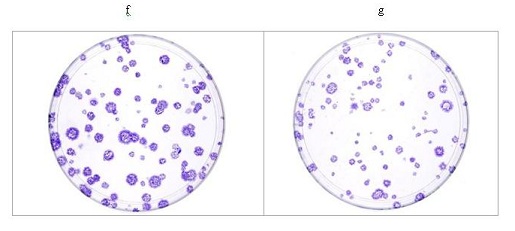 Figure 4: Colony forming unit activity of MSC grown in normoxic (f) & hypoxic conditions (g).
Figure 4: Colony forming unit activity of MSC grown in normoxic (f) & hypoxic conditions (g).
MSC exhibited a significant different morphology with shorter extensions and broader cell bodies in 5% oxygen tension environment, whereas MSC exhibited a typical spindle shaped with long cytoplasmic extension in normoxic (21% O2) environment (Figure 5) after Giemsa staining.
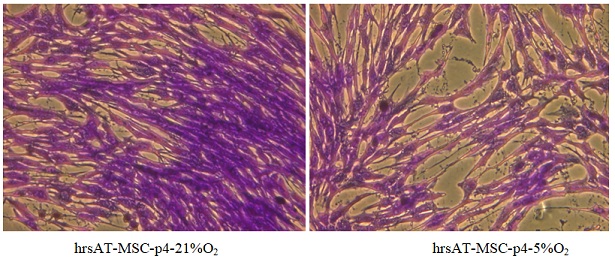 Figure 5: Morphology of MSC after Giemsa staining.
Figure 5: Morphology of MSC after Giemsa staining.
The immunophenotype with Integrin alpha 5 expressions was higher in normoxia than hypoxic conditions in both hrs AT-MSC as well as hrs UCB-MSC (Figure 6).
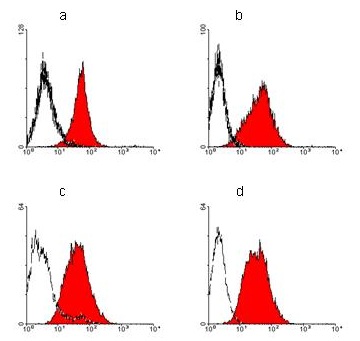 Figure 6: Expression of Integrin alfa 5 by FACS: a) hrs AT-MSC under normoxia, b) hrs AT-MSC under hypoxia, c) hrs UCB-MSC under normoxia, d) hrs UCB-MSC under hypoxia.
Figure 6: Expression of Integrin alfa 5 by FACS: a) hrs AT-MSC under normoxia, b) hrs AT-MSC under hypoxia, c) hrs UCB-MSC under normoxia, d) hrs UCB-MSC under hypoxia.
Discussion
Cell count, cell colony and cell size, cell proliferation of equine stem cells was higher in normoxic condition than hypoxia. Heterogeneity of MSC was also less than hypoxia. It can be hypothesized that the low proliferative activity of MSC in the culture reflects the severity of damage under conditions of oxidative stress during hypoxic culturing (5% O2). Cell cycle analysis revealed that more cells entered the G2/M phase in normoxic condition than hypoxic. This decrease of cells in the G2/M phase confirms the inhibitory effect of reduced oxygen concentrations on MSC proliferation. Normoxia significantly increased proliferative activity of MSC, which is consistent with published data [13]. The number of cells increased 2.1 times during culturing at d 8 under normoxic condition than hypoxic. Viability of both hrs-AT-MSC and hrs-UCB-MSC was higher in normoxia than hypoxia culture as reputed by other worker [13].
The immunophenotype with Integrin alpha 5 expressions was higher in normoxia than hypoxic conditions in both hrs AT-MSC as well as hrs UCB-MSC. Better differentiation of MSC was observed in normoxic condition of both types of MSC (hrs-AT-MSC and hrs-UCB-MSC) than in hypoxic environment [13]. The Integrin family of cell surface receptors appears to play a major role in the mediation of the cell-extracellular matrix (ECM) interacts associated with structural and functional changes in surrounding tissues. The Integrin are heterodimeric glycoprotein that are composed of an α and a β subunit. Integrin-mediated signalling is involved in a variety of cellular process such as differentiation, adhesion and migration. Integrin-mediated signalling seems to play an important role in the generation and maintenance of the osteoblast and chondrocyte differentiation in stem cells derived from bone marrow and adipose tissue [14,15]. In our study, expression of Integrin α was found more in normoxic than hypoxic conditions in both hrs AT-MSC as well as hrs UCB-MSC. Integrin α may be considered as a good immunophototyping marker for adipose tissue derived mesenchymal stem cell culture and proliferation [16].
Oxygen concentration during standard in vitro culture of primary animal cells is often not adapted to the in vivo situation. It is becoming evident that oxygen is an important regulator of stem cell biology [13]. MSC in a slowly cycling state may be more protected from DNA damage caused by errors during replication as well as from free oxygen radical species. In this respect it is not surprising that MSC do not proliferate and differentiate under reduced oxygen levels as compared to atmospheric oxygen levels. Although we have not analysed chromosomal aberration during the culture period, mutations could be favoured by high oxygen tensions during conventional culture conditions. We therefore limit the culture period in our institute for clinical application of animal MSC to four to five weeks as a safety measure. Similar proliferation, cell cycle and cell morphology were also reported by Holzwarth et al. [13] during their study on human MSC under hypoxic (5% O2) and normoxic (21% O2) conditions. It is known that low oxygen tension is involved in keeping stem cells in a quiescent state retaining their plasticity [17]. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells [18]. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression [19]. However, there are also some conflicting reports by different workers, who reported that hypoxia increased MSC proliferative activity of cell and also had no adverse effect on MSC. MSC cultured under hypoxia for 2 weeks showed increased proliferation and viability. During long term culture, hypoxia delayed phenotypic changes in MSCs, such as increased cell volume, altered morphology and the expression of senescence-associated β-gal, without altering their characteristic immunophenotype characteristics [20]. The inconsistency of these results was possibly due to number of variations in the experimental setup, including (i) use of MSC from different species, namely rodents and humans; (ii) different media compositions, in particular source and amount of growth factors; and (iii) subtle difference in the oxygen concentrations used from 8% to 1% [13].
Conclusion
The result of this study indicated that hypoxia (5% O2 tension) during in vitro culture of equine MSC derived from adipose tissue and umbilical cord blood slows down the cell numbers, cell cycle progression, cell differentiation and viability.
References
- Maiti SK, Shiva Kumar, Srivastava L, Ninu AR, Kumar N (2013) Isolation, proliferation and morphological characteristics of bone marrow derived mesenchymal stem cells from different animal species. Trends Biomater Artif Organs 27 : 29-35.
- Smith RK, Korda M, Blunn GW, Goodship AE (2003) Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J 35: 99-102.
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, et al. (2007) Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg 36: 613-622.
- Koch TG, Heerkens T, Thomsen PD, Betts DH (2007) Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol 7: 26-34.
- Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, et al. (2006) Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 24: 1613-1619.
- Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95: 9-20.
- Das R, Jahr H, Gerjo JVM, Osch V, Farrell E (2010) The role of hypoxia in bone marrow-derived mesenchymal stem cells: Consideration for regenerative medicine approaches. Tissue Engineering Part B Rev 16: 159-168.
- Csete M (2005) Oxygen in the cultivation of stem cells. Ann New York Acad Sci 1049: 1-8.
- Lennon DP, Edmison JM, Caplan AI (2001) Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochodrogenesis. J Cell Physiol 187: 345-355.
- Ivanovic Z (2009) Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol 219 : 271-275.
- Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, et al. (2006) Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 24: 2202-2208.
- Burakova LB, Anokhina EB (2007) Effect of hypoxia on stromal precursors from rat bone marrow at the early stage of culturing. Bull Expt Biol Med 143: 386-389.
- Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, et al. (2010) Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biology 11: 11.
- Goessler UR, Bugert P, Bieback K, Straeter JS, Bran G, et al. (2008) Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med 21: 271-279.
- Navarrete RO, Rodil SE, Hyzy SL, Dunn GR, Flores AA, et al. (2015) Role of Integrin subunits in mesenxhymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructures surfaces. Biomaterials 51: 69-79.
- Maiti SK, Wouters G, Spitkovsky S, Hescheler J (2021) Effect of different serums on culture and growth pattern on equine adipose derived mesenchymal stem cells (hrs-AT-MSC). Journal of Stem cell Research & Therapeutics 7: 12-16.
- D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39: 513-522.
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, et al. (2007) Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6: 745-757.
- Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, et al. (2007) Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 40: 1078-1087.
- Kim DS, Ko YJ, Lee MW, Park HJ, Park YJ, et al. (2016) Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 21: 1089-1099.
Citation: Maiti SK, Wouters G, Spitkovsky D, Hescheler J (2022) Effect of Different Oxygen Tension on Culture and Growth Pattern of Horse Adipose Tissue and Umbilical Cord Blood Derived Mesenchymal Stem Cells (hrs-AT-MSC and hrs-UCB-MSC). J Stem Cell Res Dev Ther 8: 092.
Copyright: © 2022 Swapan Kumar Maiti, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

