Effects of Low-Molecular Heparin on Pregnant Women with Factor V Mutation (GA Genotype)
*Corresponding Author(s):
Andrey Pavlovich MomotAltai Branch Fsfi National Research Center For Hematology, Ministry Of Healthcare Of The Russian Federation, Barnaul, Russian Federation
Tel:+7 3852689800,
Email:xyzan@yandex.ru
Abstract
Objective: To study the clinical laboratory results of heparin prophylaxis in women with FVL (1691) GA mutation with severe APC resistance.
Material and methods: A single-center randomized controlled study of 141 pregnant women with FVL mutation (GA genotype) with APC resistance (normalized ratio of 0.49 or less) at 7-8 weeks of gestation has been conducted. The study group consisted of 70 patients who underwent courses of heparin prophylaxis during 14 days from 7-8 weeks of gestation. 71 pregnant women who received no antenatal LMWH prophylaxis were in the control group.
Results: Prophylactic doses of LMWH decreased thrombin generation from 22 weeks of gestation: Peak thrombin by 9-11% (p <0.05) and ETP by 4-9% (p <0.05), also there was a decrease in APC resistance from 12 weeks by 9-14% (p <0.05) compared to pregnant women receiving standard prophylactic measures. Heparin prophylaxis in women with FVL (1961) GA mutation from 7-8 weeks of gestation reduced the absolute risk (ARR, Absolute Risk Reduction) of placenta-mediated pregnancy complications: PE by 29.5% [ARR: 29.5; p =0.0003; NNT: 3.4, 95% CI (2,35-6,12)], FGR by 23.8% [ARR: 29.5; p =0.0016; NNT: 4.2; 95% CI (2.8-8.7)] and PTL by 12.6% [ARR: 29.5; p =0.0242; NNT: 5.8; 95% CI (3.7-14.1)].
Keywords
ABBREVIATIONS
APC: Activated Protein ?
APS: Antiphospholipid Syndrome
AUC: Area Under Roc Curve
CER: Control Event Rate
DBP: Diastolic Blood Pressure
EER: Experimental Event Rate
ETP: Endogenous Thrombin Potential
FGR: Fetal Growth Restriction
FVL: Factor V Leiden
IL-6: Interleukin 6
LMWH: Low Molecular Weight Heparin
NNT: Number Needed To Treat
NR: Normalized Ratio
PCR: Polymerase Chain Reaction
PDONLP: Premature Detachment of a Normally Located Placenta
PE: Preeclampsia
PMC: Placenta-Mediated Complications
PTL: Preterm Labor
RL: Reproductive Loss
ROC: Receiver Operating Characteristic
RRR: Relative Risk Reduction
SBP: Systolic Blood Pressure
TGT: Thrombin Generation Test
TNF-α: Tumor Necrosis Factor-Α
U/S: Ultrasound
VTEC: Venous Thromboembolic Complications
?RR: Absolute Risk Reduction
??-EGF: Heparin-Binding Epidermal Growth Factor
INTRODUCTION
To date, Preeclampsia (PE), Fetal Growth Restriction (FGR) and Premature Detachment of a Normally Located Placenta (PDONLP) remain the main causes of perinatal and maternal morbidity/mortality [1,2]. The ambiguity of reliable data on the etiology and pathogenesis of these placenta-mediated conditions does not allow to develop a universal complex of measures for their effective prevention in the population. One of the proven risk factors for disrupting placenta formation is the genetic thrombophilias, in particular Factor V Leiden mutation [1,3,4]. This is a point mutation of the proaccelerin factor gene, accompanied by the replacement of the guanine nucleotide by adenine at position 1691 (FVLG1691A), which leads to the replacement of the amino acid Arginine (Arg =R) by the amino acid Glutamine (Gln =Q) at position 506 (FV R506Q) in the protein chain, which is the product of this gene. In this case, the polypeptide loses one of the activated protein C cleavage sites which leads to factor Va resistance to Activated Protein C (APC resistance) accompanied by an increase in thrombin generation [5-7]. The resulting imbalance can lead not only to increased coagulation potential [8-14], but also to the disorder of invasion and placentation, which in the future can manifest itself clinically in placenta-mediated complications [2,15-17]. In particular, according to available data, FVL (1691) GA increases the risk of PE by 2.19; FGR by 2.68 and PDONLP by 4.7 [4,18].
In the world practice, the use of Low Molecular Weight Heparins (LMWH) has been repeatedly considered as prevention of PE and FGR in groups at high risk for gestational complications. However, the emphasis was not on the anticoagulant properties of LMWH, but on its additional effects during the development of trophoblast [19-23]. Nevertheless, the results of studies on heparin for gestational complications prophylaxis in women with a history of gestational complications can be considered contradictory [24-32], which, apparently, is caused by different inclusion criteria and insufficient stratification of patients into risk groups, based on individual characteristics, as well as on LMWH administration method.
In a Cochrane review of 1228 women, the researchers concluded that the use of LMWH in women with an unexplained recurrent miscarriage is not justified. According to this document, the effect of LMWH on pregnancy outcomes in patients with recurrent miscarriage with underlying genetic thrombophilia has not been proven and requires further randomized controlled trials [33].
In our prospective cohort study published earlier, which included 500 women with FVL (1691) GA mutation, it was shown that APC resistance ≤0.49 [Normalized Ratio (NR)] can be considered as a prognostic marker for PE (Area Under Roc Curve (AUC)-0,839, p <0.0001) and FGR (Area Under The Roc Curve (AUC)-0.867, p <0.0001) with the greatest accuracy at 7-8 weeks of gestation [34]. We have found no studies investigating the effectiveness of LMWH in preventing placenta-mediated complications considering APC resistance, except for a publication that includes a small sample of 4 pregnant women [35], which was the reason for the present study.
OBJECTIVE
To study the clinical laboratory results of heparin prophylaxis in women with FVL (1691) GA mutation with severe APC resistance.
METHODS
Study population
• Inclusion criteria: FVL (1691) GA with APC resistance ≤0.49; normal singleton pregnancy, occurring in the natural cycle; gestational age of 7-8 weeks.
• Exclusion criteria: FVL (1691) GG/AA genotype; genital organ anomalies; multiple pregnancies; pregnancy, resulting from assisted reproductive technologies; extragenital disease in the stage of decompensation; autoimmune diseases, including antiphospholipid syndrome; chromosomal aberrations in spouses.
The study was approved by the local ethical committee of FSBEI HE ASMU of the Ministry of Health of the Russian Federation (Protocol No. 5 of 25.06.2009).
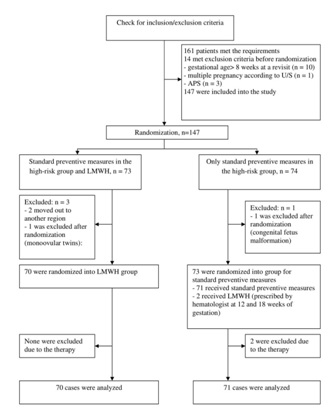
In total, during the period from 2015 to 2017, 161 patients, who met the inclusion criteria, were selected. All patients had an intermediate risk of Venous Thromboembolic Complications (VTEC) and, according to the recommendations of the world community, did not need antenatal thromboprophylaxis [36,37]. At the stage of group formation, 20 patients left the study (Figure 1): 14 had exclusion criteria before randomization; 4 were excluded in the first trimester of pregnancy (2 went to another region, 1 had monoovular twins and 1 had fetal malformations), and 3 patients left the control group at the observation stage, because they needed LMWH upon the hematologist’s prescription due to DVT episodes. As a result, 141 patients entered the study. The main group consisted of 70 pregnant women (mean age 30.2?4.7) who underwent heparin prophylaxis from 7-8 weeks of gestation. 71 pregnant women were in the control group (mean age 30.3?3.9), where no antenatal LMWH prophylaxis was given. Block randomization was used [38,39].
Blood collection
Laboratory assays
APC resistance was measured with the “Factor V-PC-test” (Technology-Standard, Russia), an analog of the corresponding set of reagents produced by Siemens, Germany. To study the thrombin Fluoroskan Ascent “Thermo Fisher Scientific” (Finland) with “Thrombinoscope 3.0.0.26” software was used. Coagulation of the test plasma was performed in the presence of 5.0 pmol of tissue factor and 4 μmol of phospholipids (PPP-Reagent 5 pM, Thrombin Calibrator, FluCa-Kit).
Molecular genetic testing of the gene alleles of Factor V Leiden (F5 Arg 506 Gln) was performed using Real-time PCR with reagents from “Litekh” SPA (Russia). The material for the study was human genomic DNA taken from peripheral blood leukocytes.
Heparin prophylaxis
Noteworthy is that in both groups, patients at high risk of preeclampsia (52.9% (37 of 70) in the study group and 42.1% (32 of 71) in the control group, p >0.05) received prophylactic doses of acetylsalicylic acid (75 mg per day) according to the clinical protocol [41].
The endpoints determining the efficacy of LMWH were: The number of cases of moderate/severe PE, FGR and PDONLP episodes and the number of induced Preterm Labor (PTL).
Preeclampsia was determined according to International Consensus Criteria: Systolic Blood Pressure (SBP) ≥140 mmHg and/or Diastolic Blood Pressure (DBP) ≥90 mmHg; in women with initial hypotension, an increase in SBP by 30 mmHg and/or DBP by 15 mmHg compared to the initial one (arterial blood pressure in the first trimester of pregnancy), accompanied by proteinuria: A daily protein loss of 0.3 g/l and more, any proteinuria recorded in a single portion of urine [42]. Fetal growth restriction was defined as a condition in which the fetal body weight and/or fetal abdomen circumference is below 10% for a given gestational age and/or the morphological maturity index lags 2 or more weeks from the true gestational age [43]. The induced preterm labor was the delivery at 22+0 to 36+6 weeks, performed due to mother’s critical condition (increasing severity of somatic diseases, pregnancy complications) and/or fetus (progressive decline, antenatal fetal death).
Statistical calculation
RESULTS
At the first stage of the study, the clinical characteristics of patients were studied according to the traditionally considered risk factors for development of placenta-mediated conditions. As a result, it was shown that both groups were representative in age, thrombotic and reproductive history and somatic pathology (Table 1).
| Somatic and Reproductive History of the Groups | LMWH “+” n=70 | LMWH “-” n=71 | ? | ||
| Hypertensive heart disease | 20 | 28.6% | 17 | 23.9% | 0.624 |
| Varicose disease of lower extremities | 34 | 48.6% | 31 | 43.7% | 0.584 |
| Excess body weight (BMI >25) | 29 | 41.4% | 28 | 39.4% | 0.241 |
| Age >35 years old | 10 | 14.3% | 16 | 22.5% | 0.091 |
| History of thrombotic complications | 20 | 28.6% | 18 | 25.4% | 0.118 |
| History of 3 or more reproductive losses | 12 | 17.1% | 9 | 12.7% | 0.742 |
| History of an antenatal loss | 7 | 10.0% | 5 | 7.0% | 0.626 |
| History of preeclampsia | 17 | 24.3% | 15 | 21.1% | 0.447 |
| History of FGR | 15 | 21.4% | 13 | 18.3% | 0.464 |
| Premature detachment of the placenta | 3 | 4.3% | 5 | 7.0% | 0.701 |
| Spotting in 1st trimester, requiring hospitalization | 12 | 17.1% | 11 | 15.5% | 0.265 |
Table 1: Somatic and reproductive history of patients with Factor V Leiden [FVL (1691) GA] of the groups.Abbreviations: LMWH “+”-patients who received heparin prophylaxis; LMWH “-”-patients without heparin prophylaxis.
Further analysis showed that patients with FVL (1691) GA had elevated values of thrombin generation at 7-8 weeks (ETP median by 1.3: 1999 nmol × min vs 1542 nmol × min, p <0.0001; peak thrombin by 1.5: 423 nmol/l vs 290 nmol/l, p <0.0001) in comparison to the physiological norm, which had been published earlier [44]. As pregnancy developed, these values acquired statistically significant differences depending on whether heparin prophylaxis was performed or not (Figures 2 and 3). 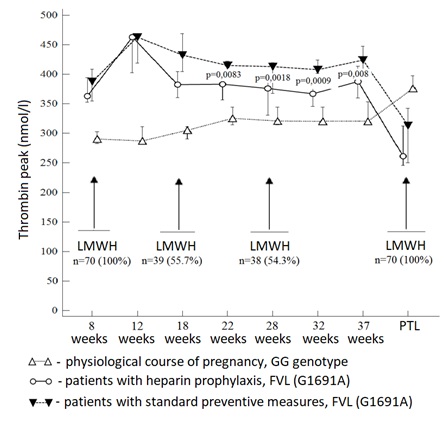 Figure 2: Dynamics of peak thrombin median in patients with FVL (1961) GG genotype and FVL (1961) GA genotype, depending on the heparin prophylaxis.
Figure 2: Dynamics of peak thrombin median in patients with FVL (1961) GG genotype and FVL (1961) GA genotype, depending on the heparin prophylaxis. 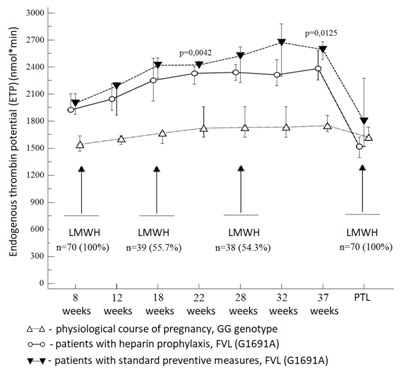 Figure 3: Dynamics of endogenous thrombin potential median in patients with FVL (1961) GG genotype and FVL (1961) GA genotype, depending on whether heparin prophylaxis was carried out or not. In particular, a decrease in thrombin generation due to LMWH intake was determined from 22 weeks of gestation: Peak thrombin by 9-11% and ETP by 4-9%.
Figure 3: Dynamics of endogenous thrombin potential median in patients with FVL (1961) GG genotype and FVL (1961) GA genotype, depending on whether heparin prophylaxis was carried out or not. In particular, a decrease in thrombin generation due to LMWH intake was determined from 22 weeks of gestation: Peak thrombin by 9-11% and ETP by 4-9%.
Along with a predictable response of TGT main parameters to the LMWH, the decrease in APC resistance (NR) was unexpected (Table 2).
| Study Points | LMWH “-” n=70 | LMWH “+” n=71 | Statistical Values | ||||
| Me | 95% CI | Me | 95% CI | Mann-Whitney U |
Test statistic Z |
? | |
| 8 weeks | 0.47 | 0.45-0.48 | 0.47 | 0.46-0.48 | 2398 | 0.366 | 0.7142 |
| 12 weeks | 0.46 | 0.44-0.48 | 0.5 | 0.47-0.51 | 329 | 3.424 | 0.0006 |
| 18 weeks | 0.46 | 0.44-0.48 | 0.5 | 0.46-0.51 | 421 | 3.828 | 0.0001 |
| 22 weeks | 0.45 | 0.42-0.46 | 0.51 | 0.49-0.52 | 173.5 | 4.96 | <0.0001 |
| 28 weeks | 0.44 | 0.41-0.45 | 0.5 | 0.48-0.51 | 111.5 | 4.26 | <0.0001 |
| 32 weeks | 0.44 | 0.42-0.46 | 0.49 | 0.45-0.50 | 162.5 | 2.755 | 0.0059 |
| 37 weeks | 0.5 | 0.49-0.51 | 0.5 | 0.49-0.52 | 136 | 2.058 | 0.0396 |
| PTL | 0.5 | 0.49-0.51 | 0.52 | 0.47-0.53 | 147.5 | 0.424 | 0.6717 |
Table 2: NR median values depending on whether heparin prophylaxis was carried out or not at different stages of pregnancy in women with FVL (1961) GA.
From the data presented, it can be seen that the APC resistance during LMWH intake decreased statistically significantly from 12 weeks of pregnancy, in contrast to the results in the control group (Figure 4). 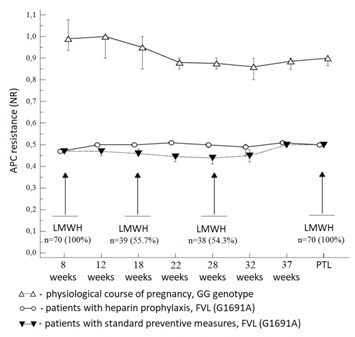
Figure 4: Dynamics of the NR median reflecting APC resistance in patients with FVL (1961) GG and GA genotypes, depending on whether heparin prophylaxis was carried out or not.
In general, due to heparin prophylaxis, a decrease in APC resistance by 9-14% (p <0.05) was found in comparison to pregnant women receiving standard preventive measures.
Further, associations between heparin prophylaxis and the incidence of placenta-mediated complications were studied (Table 3). It was found that in the group of women who received LMWH, starting from 7-8 weeks, the number of PE cases decreased, in comparison to the standard care group, by 29.5%, FGR by 23.8%, induced PTL by 12.6% and PDONLP by 5.6%.
As a matter of end points, the values determining the degree of effectiveness of drug intervention were calculated in accordance to the accepted practice with p
According to the presented data, in the study group there is a statistically significant ARR of PE development (p =0.0003), FGR (p =0.0016) and induced PTL (p =0.0242). In particular, the pregnancy ended with an induced PTL in the study group in two patients (2.9% of 70), including one case of PDONLP (33 weeks) and one antenatal fetal death (32 weeks). In the control group, 11 pregnant women had induced PTL (15.5% of 71, 24-34 weeks), including 3 cases of severe PE, 3 cases of PONRP, 3 cases due to threatening intrauterine fetal asphyxia and 2 cases due to antenatal fetal death.
DISCUSSION
Part 1
CONCLUSION
1. Not only a more pronounced increase in thrombin generation during pregnancy in FVL (1691) GA patients (compared to the normal genotype), but also a decrease in thrombin generation due to heparin prophylaxis were found out.
2. Prophylactic doses of LMWH decrease APC resistance, which is probably related to the effect on the causes that determine its acquired component, which is not genetically determined.
3. The use of LMWH in a prophylactic dose in FVL (1961) GA patients from 7-8 weeks reduces the absolute risk of placenta-mediated pregnancy complications, including PE (by 29.5%), FGR (by 23.8%) and induced PTL (by 12.6%).
REFERENCES
- Brosens I, Pijnenborg R, Vercruysse L, Romero R (2011) The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 204: 193-201.
- Mastrolia SA, Mazor M, Loverro G, Klaitman V, Erez O (2014) Placental vascular pathology and increased thrombin generation as mechanisms of disease in obstetrical syndromes Peer J 18: 653.
- Di Renzo GC (2009) The great obstetrical syndromes. J Matern Fetal Neonatal Med 22: 633-635.
- Wang X, Bai T, Liu S, Pan H, Wang B (2014) Association between thrombophilia gene polymorphisms and preeclampsia: A meta-analysis. PLoS One 9: 100789.
- Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, et al. (1994) Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 369: 64-67.
- Dissanayake VH, Weerasekera LY, Gammulla CG, Jayasekara RW (2009) Prevalence of genetic thrombophilic polymorphisms in the Sri Lankan population--implications for association study design and clinical genetic testing services. Exp Mol Pathol 87: 159-162.
- Rosendaal FR, Reitsma PH (2009) Genetics of venous thrombosis. J Thromb Haemost 7: 301-304.
- Hézard N, Bouaziz-Borgi L, Remy MG, Nguyen P (2006) Utility of thrombin-generation assay in the screening of factor V G1691A (Leiden) and prothrombin G20210A mutations and protein S deficiency. Clin Chem 52: 665-670.
- Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C (2006) Use of calibrated automated thrombinography +/- thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost 96: 562-567.
- Couturaud F, Duchemin J, Leroyer C, Delahousse B, Abgrall JF, et al. (2008) Thrombin generation in first-degree relatives of patients with venous thromboembolism who have factor V Leiden. A pilot study. Thromb Haemost 99: 223-228.
- Baglin T (2011) Using the laboratory to predict recurrent venous thrombosis. Int J Lab Hematol 33: 333-342.
- Sonnevi K, Tchaikovski SN, Holmström M, Rosing J, Bremme K, et al. (2011) Thrombin generation and activated protein C resistance in the absence of factor V Leiden correlates with the risk of recurrent venous thromboembolism in women aged 18-65 years. Thromb Haemost 106: 901-907.
- Segers O, Simioni P, Tormene D, Castoldi E (2014) Influence of single nucleotide polymorphisms on thrombin generation in factor V Leiden heterozygotes. Thromb Haemost 111: 438-446.
- Kojima T, Takagi A, Murata M, Takagi Y (2015) [Antithrombin resistance: A new mechanism of inherited thrombophilia]. Rinsho Ketsueki 56: 632-638.
- Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K (1997) Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol 177: 402-405.
- Many A, Schreiber L, Rosner S, Lessing JB, Eldor A, et al. (2001) Pathologic features of the placenta in women with severe pregnancy complications and thrombophilia. Obstet Gynecol 98: 1041-1044.
- Gogia N, Machin GA (2008) Maternal thrombophilias are associated with specific placental lesions. Pediatr Dev Pathol 11: 424-429.
- Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J (2008) Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American college of chest physicians evidence-based clinical practice guidelines, (8th Edn). Chest 133: 844-886.
- Quenby S, Mountfield S, Cartwright JE, Whitley GS, Vince G (2004) Effects of low-molecular-weight and unfractionated heparin on trophoblast function. Obstet Gynecol 104: 354-361.
- Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, et al. (2005) Heparin and aspirin attenuate placental apoptosis in vitro: Implications for early pregnancy failure. Am J Obstet Gynecol 192: 23-30.
- Bose P, Black S, Kadyrov M, Bartz C, Shlebak A, et al. (2004) Adverse effects of lupus anticoagulant positive blood sera on placental viability can be prevented by heparin in vitro. Am J Obstet Gynecol 191: 2125-2131.
- Hills FA, Abrahams VM, González-Timón B, Francis J, Cloke B, et al. (2006) Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod 12: 237-243.
- Ganapathy R, Whitley GS, Cartwright JE, Dash PR, Thilaganathan B (2007) Effect of heparin and fractionated heparin on trophoblast invasion. Hum Reprod 22: 2523-2527.
- Gris JC, Mercier E, Quéré I, Lavigne-Lissalde G, Cochery-Nouvellon E, et al. (2004) Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 103: 3695-3699.
- Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, et al. (2008) Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. J Obstet Gynaecol 28: 280-284.
- Rey E, Garneau P, David M, Gauthier R, Leduc L, et al. (2009) Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: A pilot randomized controlled trial. J Thromb Haemost 7: 58-64.
- Mastrolia SA, Novack L, Thachil J, Rabinovich A, Pikovsky O, et al. (2016) LMWH in the prevention of preeclampsia and fetal growth restriction in women without thrombophilia. A systematic review and meta-analysis. Thromb Haemost 116: 868-878.
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, et al. (2014) Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): A multinational open-label randomized trial. Lancet 384: 1673-1683.
- Martinelli I, Ruggenenti P, Cetin I, Pardi G, Perna A, et al. (2012) Heparin in pregnant women with previous placenta-mediated pregnancy complications: A prospective, randomized, multicenter, controlled clinical trial. Blood 119: 3269-3275.
- Haddad B, Winer N, Chitrit Y, Houfflin-Debarge V, Chauleur C, et al. (2016) Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: A randomized controlled trial. Obstet Gynecol 128: 1053-1063.
- Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin-Papunen L, et al. (2011) Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia. HABENOX: A randomized multicenter trial. Thromb Haemost 105: 295-301.
- Kaandorp SP, Goddijn M, van der Post JA, Hutten BA, Verhoeve HR, et al. (2010) Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 362: 1586-1596.
- de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S (2014) Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev 7.
- Nikolaeva MG, Momot AP, Serdyuk GV, Elykomov VA, Momot KA, et al. (2018) APC-resistance associated with factor V leiden gene mutation (genotype GA): Clinical occurrence in pregnancy. Thrombosis, hemostasis and rheology 73: 47-54.
- de Vries JI, van Pampus MG, Hague WM, Bezemer PD, Joosten JH, et al. (2012) Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J Thromb Haemost 10: 64-72.
- Royal College of Obstetricians and Gynaecologists (2015) Thromboembolic disease in pregnancy and the puerperium: Acute Management. Green-top Guideline No. 37b. Royal College of Obstetricians and Gynaecologists, London, UK. Pg no: 1-32.
- Bates SM, Middeldorp S, Rodger M, James AH, Greer I (2016) Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 41: 92-128.
- Randelli P, Arrigoni P, Lubowitz JH, Cabitza P, Denti M (2008) Randomization procedures in orthopaedic trials. Arthroscopy 24: 834-838.
- Bridgman S, Engebretsen L, Dainty K, Kirkley A, Maffulli N, et al. (2003) Practical aspects of randomization and blinding in randomized clinical trials. Arthroscopy 19: 1000-1006.
- Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, et al. (2003) Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 33: 4-15.
- Hypertensive disorders during pregnancy, delivery and the puerperium period. Preeclampsia. Eclampsia. Clinical recommendations (protocol of treatment) the letter of the Ministry of Health of the Russian Federation, Russia.
- Tranquilli AL (2013) Introduction to ISSHP new classification of preeclampsia. Pregnancy Hypertens 3: 58-59.
- Royal College of Obstetricians and Gynecologists (2015) The investigation and management of the small-for-gestational-age fetus. Green-top Guideline N 31. Royal College of Obstetricians and Gynaecologists, London, UK.
- Momot AP, Semenova NA, Belozerov DE, Trukhina DA, Kudinova IY (2016) The dynamics of the hemostatic parameters in physiological pregnancy and after delivery. J Hematol Blood Transfus Disord 3: 005.
- Joly B, Barbay V, Borg JY, Le Cam-Duchez V (2013) Comparison of markers of coagulation activation and thrombin generation test in uncomplicated pregnancies. Thromb Res 132: 386-391.
- Spiezia L, Bogana G, Campello E, Maggiolo S, Pelizzaro E, et al. (2015) Whole blood thromboelastometry profiles in women with preeclampsia. Clin Chem Lab Med 53: 1793-1798.
- Rosenkranz A, Hiden M, Leschnik B, Weiss EC, Schlembach D, et al. (2008) Calibrated automated thrombin generation in normal uncomplicated pregnancy. Thromb Haemost 99: 331-337.
- Selmeczi A, Roach RE, Móré C, Batta Z, Hársfalvi J, et al (2015) Thrombin generation and low-molecular-weight heparin prophylaxis in pregnant women with thrombophilia. Thromb Haemost 113: 283-289.
- Lattová V, Procházka M, Procházková J, Ulehlová J, Slavík L, et al. (2013) [Preeclampsia and thrombin generation test]. Ceska Gynekol 78: 466-472.
- Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, et al. (2018) The pattern and magnitude of “in vivo thrombin generation” differ in women with preeclampsia and in those with SGA fetuses without preeclampsia. J Matern Fetal Neonatal Med 31: 1671-1680.
- Chui AKL, Gunatillake TN, Ignjatovic V, Monagle PT, Murthi P, et al. (2017) Antiangiogenic effects of decorin restored by unfractionated, low molecular weight, and nonanticoagulant heparins. Blood Adv 1: 1243-1253.
- Dahlbäck B, Carlsson M, Svensson PJ (1993) Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: Prediction of a cofactor to activated protein C. Proc Natl Acad Sci USA 90: 1004-1008.
- Clark P, Sattar N, Walker ID, Greer IA (2001) The Glasgow Outcome, APCR and Lipid (GOAL) pregnancy study: Significance of pregnancy associated activated protein C resistance. Thromb Haemost 85: 30-35.
- Martinelli I, Legnani C, Bucciarelli P, Grandone E, De Stefano V, et al. (2001) Risk of pregnancy-related venous thrombosis in carriers of severe inherited thrombophilia. Thromb Haemost 86: 800-803.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, et al. (1998) Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 79: 1166-1170.
- Bellart J, Gilabert R, Fontcuberta J, Borell M, Minalles RM, et al. (1997) Fibrinolysis changes in normal pregnancy. J Perinat Med 25: 368-372.
- McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, et al. (2017) Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension 69: 180-188.
- Clark P, Walker ID, Langhorne P, Crichton L, Thomson A, et al. (2010) SPIN (Scottish Pregnancy Intervention) study: A multicenter, randomized controlled trial of low-molecular-weight heparin and low-dose aspirin in women with recurrent miscarriage. Blood 115: 4162-4167.
- Pasquier E, de Saint Martin L, Bohec C, Chauleur C, Bretelle F, et al. (2015) Enoxaparin for prevention of unexplained recurrent miscarriage: A multicenter randomized double-blind placebo-controlled trial. Blood 125: 2200-2205.
- Groom KM, McCowan LM, Mackay LK, Lee AC, Said JM, et al. (2017) Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: A randomized trial. Am J Obstet Gynecol 216: 296.
- Singh S, Sinha R, Kaushik M (2016) Prophylactic low molecular weight heparin improving perinatal outcome in non-thrombophilic placental-mediated complications. J Obstet Gynaecol India 66: 436-440.
- Di Simone N, Di Nicuolo F, Sanguinetti M, Ferrazzani S, D’Alessio MC, et al. (2007) Low-molecular weight heparin induces in vitro trophoblast invasiveness: Role of matrix metalloproteinases and tissue inhibitors. Placenta 28: 298-304.
- D’Ippolito S, Di Nicuolo F, Marana R, Castellani R, Stinson J, et al. (2012) Emerging nonanticoagulant role of low molecular weight heparins on extravillous trophoblast functions and on heparin binding-epidermal growth factor and cystein-rich angiogenic inducer 61 expression. Fertil Steril 98: 1028-1036.
- Chen Y, Wu XX, Tan JP, Liu ML, Liu YL, et al. (2012) Effects of low molecular weight heparin and heparin-binding epidermal growth factor on human trophoblast in first trimester. Fertil Steril 97: 764-770.
- Bolnick AD, Bolnick JM, Kohan-Ghadr HR, Kilburn BA, Pasalodos OJ, et al. (2017) Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor. Hum Reprod 32: 1218-1229.
- Middeldorp S (2007) Pregnancy failure and heritable thrombophilia. Semin Hematol 44: 93-97.
- Nishiguchi T, Kobayashi T (2005) Antiphospholipid syndrome: Characteristics and obstetrical management. Curr Drug Targets 6: 593-605.
- Zenerino C, Nuzzo AM, Giuffrida D, Biolcati M, Zicari A, et al. (2017) The HMGB1/RAGE Pro-inflammatory axis in the human placenta: Modulating effect of low molecular weight heparin. Molecules 22.
Citation: Momot AP, Nikolaeva MG, Zainulina MS, Momot KA, Yasafova NN (2018) Effects of Low-Molecular Heparin on Pregnant Women with Factor V Mutation (GA Genotype). J Hematol Blood Transfus Disord 5: 20.
Copyright: © 2018 Andrey Pavlovich Momot, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.