
Efficacy and Safety of Suvorexant in a Palliative Care Unit: A Retrospective, Single-Center Study
*Corresponding Author(s):
Takefumi NishimotoHakodate Goryoukaku Hospital, 38-3 Goryoukaku-cho, Hakodate City, Hokkaido, Japan
Tel:+81-138-51-2295,
Email:takefumin@a181.org
Abstract
- Objectives
The frequent occurrence of insomnia among patients in Palliative Care Units (PCUs) due to physical and/or psycho-social distress is an important clinical issue. Suvorexant is a new hypnotic agent that inhibits system arousal by selectively blocking the wake-promoting neurotransmitter orexin. Suvorexant reportedly increases time in slow-wave sleep, decreases the incidence of delirium does not cause muscle relaxation, unlike benzodiazepines (BZDs, gamma-aminobutyric acid A receptor agonists). We hypothesized that suvorexant is more effective and safer for the treatment of insomnia than BZDs in the context of PCUs.
- Methods
We retrospectively researched the prescription status of hypnotics and their efficacy and safety at the PCU of Kitami Red-Cross Hospital.
- Results
Suvorexant was more clinically effective (85.7% versus 50.0%), with a lower incidence of delirium (14.3% versus 25.0%), than BZDs.
- Significance of results
Higher levels of orexin caused by inflammation are suspected to lead to insomnia in advanced cancer patients. In this context, suvorexant, an orexin receptor antagonist, is assumed to show favorable hypnotic activity via its anti-inflammatory effect. Additionally, suvorexant regulates the sleep–wake cycle via the orexin system in the lateral hypothalamus, which might assist the treatment and prevention of delirium. Thus, suvorexant could be a rational and favorable treatment option to improve cancer-related insomnia in PCUs.
Keywords
GABAA Receptor Agonists; Insomnia; Orexin; Palliative Care Unit; Suvorexant
Introduction
Sleep disturbance is a common and distressing problem in palliative care settings, due to devastating physical (e.g., pain, dyspnea, fatigue) [1] and psychosocial distress [2], as well as environmental factors. Insomnia and changes in sleep status reportedly occur in 47% to 96% of patients in palliative care units (PCUs) [1-3] and are often refractory. Currently, benzodiazepines (BZDs, gamma-aminobutyric acid receptor A agonists), are most commonly used for the pharmacological treatment of sleep-related complaints, even in PCU patients. However, the Cochrane Database Systematic Review has not reported any unequivocal clinical evidence on BZDs for the treatment of insomnia in palliative care contexts [4]. In addition to insomnia, BZDs are used for controlling anxiety, dyspnea and severe pain in PCUs [5]. However, no favorable effect has been observed in patients with lower back pain following treatment with diazepam and a placebo [6]. In this context, 39% of physicians claim that BZDs are overused in their own hospice [5]. Generally, in elderly patients, BZDs can cause various adverse effects, including drowsiness, sedation, cognitive dysfunction, muscle relaxation, thereby contributing to falls and fractures [7-9]. Additionally, BZDs lead to decreased deep sleep and precipitate delirium [10-12]. Therefore, we assume that the efficacy of BZD use may be limited for cancer-related insomnia.
Suvorexant, a reversible dual orexin receptor antagonist, was approved in 2014 by the U.S. Food & Drug Administration for the treatment of insomnia [13]. Orexins are neuropeptides secreted by lateral hypothalamic neurons; they are involved in regulating the sleep–wake cycle and play a role in keeping individuals awake [14]. Suvorexant binds reversibly to the receptors of orexins and inhibits the activation of the arousal system, thereby facilitating sleep induction and maintenance [15]. Generally, the clinical effect of suvorexant against insomnia is not inferior to that of BZDs with respect to total sleep duration, sleep latency [7,16] and time in slow-wave sleep [11] and is favored to avoid muscle relaxation [16]. However, the orexin levels in the brains of rats subjected to inflammation, such as pancreatitis, are higher than those in the brains of rats without inflammation [17]. Additionally, various immunological and inflammatory reactions have been associated with cancer [18]. Higher levels of orexin are suspected to induce insomnia among advanced cancer patients.
Objective
We, assumed that the use of the orexin antagonist suvorexant is more rational for treating cancer-related insomnia in palliative care settings than that of BZDs. Therefore, we studied the applicability of suvorexant in treating insomnia in a palliative care setting. To this end, the efficacy and safety of suvorexant and BZDs against insomnia were retrospectively reviewed at the PCU of Kitami Red-Cross hospital, Hokkaido, Japan.
Methods
From June to November 2015, 73 patients who had already finished their chemotherapy were admitted to the PCU, where they exclusively received palliative care. Among these patients, 31 were administered hypnotics for insomnia. Six patients who had already been taking psychotropic medication, including antipsychotics for mental illnesses, were excluded. Finally, a total of 25 patients (mean age = 72.1 ± 12.8; sex (M/F) = 14/11) with single hypnotic treatment were enrolled in this study (Table 1).
|
|
Suvorexant |
BZDs |
P |
|
Number (female) |
7 (4) |
16 (7) |
> 0.05* |
|
Age, years |
75.6 ± 12.6 |
70.6 ± 12.6 |
> 0.05# |
|
Administration, fixed/single |
4/3 |
9/7 |
- |
Table 1: Demographics BZDs: benzodiazepines, gamma aminobutyric acid A receptor agonist.*: Chi-square test, #: Mann-Whitney U test.
The efficacy of hypnotics for insomnia was evaluated by the nurses on the night shift and the patients themselves. Practically, when both nurses and the patient judged that the patient “slept well” after the use of hypnotics, we decided the treatment was “effective,” and “ineffective” otherwise. We additionally examined the incidence of delirium and falls. The statistical analysis was performed using Bell Curve for Excel Version:3.20 (Social Survey Research Information Co., Ltd., Shinjuku-ku, Tokyo, Japan). The group differences (Suvorexant versus BZDs) of the demographic data were analyzed using the Mann-Whitney U test. The efficacy and incidence of delirium related to both BZDs and suvorexant were investigated using the Chi-square test. A p value of < 0.05 indicated statistical significance. This study was conducted in line with the Declaration of Helsinki and with the approval of the Ethics Committee of Kitami Red-Cross Hospital. We have orally presented this study at The 29th Annual Meeting of the Japan Psycho-Oncology Society.
Results
Patient demographics are shown in Table 1. Sixteen patients were administered BZDs (zolpidem tartrate, 8 patients; brotizolam, 3 patients; rilmazafone hydrochloride, 2 patients; zopiclone, 1 patient; etizolam, 1 patient; and flunitrazepam, 1 patient; (figure 1). Seven patients were administered suvorexant, 2 were administered ramelteon. No significant differences were identified in terms of age and sex between the groups (Table 1). Suvorexant showed significantly higher efficacy in treating insomnia than BZDs (85.7% [6 patients] versus 50.0% [8 patients]; (Table 2). Patients who were treated with BZDs showed a significantly higher rate of drug-related delirium than those treated with suvorexant (25.0% [4 patients] versus 14.3% [1 patient]; (Table 3). All patients administered suvorexant and 16 patients administered a BZD died. The number of days these patients spent in the PCU was not significantly different between groups (Table 4).
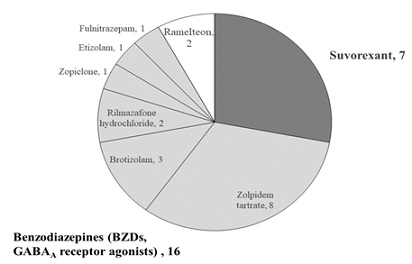 Figure 1: Hypnotics administered for PCU patients with insomnia. Dark gray: suvorexant, 7 patients. Light gray: benzodiazepines, 16 patients. White: ramelteon, 2 patients.
Figure 1: Hypnotics administered for PCU patients with insomnia. Dark gray: suvorexant, 7 patients. Light gray: benzodiazepines, 16 patients. White: ramelteon, 2 patients.
|
|
Suvorexant |
BZDs |
P |
|
Number of effective/ineffective cases |
6/1 |
8/8 |
< 0.05* |
|
% |
85.7 |
50.0 |
- |
Table 2: Effectiveness BZDs: benzodiazepines, gamma aminobutyric acid A receptor agonists. *: Chi-square test, Effective: nurses observe “the patient slept well” and the patient felt they “slept well.”
|
|
Suvorexant |
BZDs |
P |
|
Number of delirium cases |
1 |
4 |
< 0.05* |
|
% |
14.3 |
25.0 |
- |
Table 3: Delirium related to the administration of hypnotics. BZDs: benzodiazepines, gamma aminobutyric acid A receptor agonist. *: Chi-square test, Delirium: disturbance of consciousness after administration hypnotics within 24 hours.
|
|
Suvorexant |
BZD |
P |
|
Number of deaths |
7 |
11 |
- |
|
Number of days |
42.7 ± 57.1 |
19.7 ± 15.5 |
> 0.05# |
Table 4: Number of days in PCU until death. BZDs: benzodiazepines, gamma aminobutyric acid A receptor agonist. #: Mann-Whitney U test.
Discussion
In PCUs, sleep disturbance is a common and distressing problem. BZDs are conventionally used for psychologically induced insomnia [5] even in elderly subjects, despite the various adverse effects, such as falls and precipitating delirium. In this study, the efficacy and safety of suvorexant and BZD prescriptions for insomnia were retrospectively compared for PCU patients. Suvorexant was shown to be more effective in the treatment of insomnia, with a lower incidence of delirium, than BZDs.
On one hand, in palliative care settings, the Cochrane Database Systematic Review reported no clinical evidence for BZDs in the treatment of insomnia [4]. On the other hand, suvorexant reportedly increases total sleep duration and decreases sleep latency [16] without muscle relaxation or cognitive decline [19]. Our results showed that the efficacy of suvorexant (85.7%) was a little higher than that observed in the Japanese post-marketing drug-use survey (73.2%) [20]. This may be because our patients developed insomnia as a result of cancer-related inflammation. Physical pain (e.g., pain, dyspnea, fatigue) plays a crucial role in the physiobiological basis of insomnia in patients in PCUs. A survey of patients with chronic pain reported that 38% of patients use BZDs not only for sleep but also for muscle relaxation, anxiety and pain relief [21]. Moreover, another study on BZDs failed to show a significant improvement in the sleep of individuals with chronic pain conditions [22], suggesting that the efficacy of BZDs for insomnia is influenced by patients’ physical condition. For instance, midazolam is superior to morphine in controlling baseline and breakthrough dyspnea, especially in moderate or severe cases [23]. However, in our study, no patients with severe dyspnea were recruited and pain was controlled with opioids and non-opioids.
In terms of the pathophysiology of cancer-related insomnia, Kwekkeboom et al. noted significant associations of inflammation with cancer symptoms, inducing pain, fatigue, sleep disturbance [24]. For example, orexin expression in tumor tissues of gastric cancer patients is significantly upregulated compared with that in chronic gastritis patients and the control group [25]. Hamasaki et al. found significantly higher levels of orexin in the brain of rats with pancreatitis than in the healthy controls [17]. Thus, our results suggest that suvorexant improves insomnia by suppressing the elevated orexin-induced inflammation related to cancer. Additionally, slow-wave sleep reportedly decreases the fatigue associated with inflammatory activations [26]. Krasnow and Marks claimed that dysfunctions in orexin neural activity are associated with fatigue in patients with cachexia [27]. Therefore, Suvorexant may not only improve insomnia [11] but also the fatigue related to cancer inflammation. Some PCU-related environmental issues, such as noises, disturb sleep [1]. Clinically, Drake et al. reported that waking after sleep onset decreases in patients treated with suvorexant compared with those in the placebo group [28]. Suvorexant objectively increases theta band activity during sleep, providing a subjective feeling of deep sleep [11]. Additionally, Seol et al. showed impaired physical/cognitive performance upon forced awakening from sleep while using brotizolam, which was not observed in patients administered suvorexant [19]. Therefore, suvorexant may be more effective and safer than BZDs for sleep interrupted by environmental factors.
In this study, BZDs induced a higher incidence of delirium than suvorexant (25.0% versus 14.3%). BZD-induced delirium would result from the transient thalamic dysfunction caused by exposure to medications that interfere with the central glutamatergic, GABAergic, dopaminergic, cholinergic pathways [29]. However, Hatta et al. and Masuyama et al. showed the preventive effects of suvorexant on delirium, suggested that the antagonism of suvorexant against inflammation-induced elevated orexin may result in the prevention of delirium [30,31]. Falls were not observed in our study, even in the BZD cases. This may be because patients were observed carefully, all found it difficult to move unaided (the Performance Status of almost all patients was 4).
Limitations
This study had several limitations. For instance, our results are from a retrospective, single-center design with a small sample size. Additionally, the lack of quantitative measurement for sleep needs to be considered. However, the subjective feeling of sleep is assumed to be prioritized in a palliative care setting.
Conclusion
The use of suvorexant for insomnia in PCUs may be favorable compared with BZD use in terms of its biological rationality and safety. Further research in the form of a prospective randomized controlled trial with a larger sample size may confirm our findings.
Acknowledgment
We thank Dr. Ikuo Gomyou, the chief of the Department of Palliative Care and the staff of the palliative care unit at Kitami Red-Cross Hospital.
Conflicts of Interest
None.
Funding Statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
References
- Renom-Guiteras A, Planas J, Farriols C, Mojal S, Miralles R, et al. (2014) Insomnia among patients with advanced disease during admission in a palliative care unit: a prospective observational study on its frequency and association with psychological, physical and environmental factors. BMC Palliat Care 13: 40.
- Akechi T, Okuyama T, Akizumi N, Shimizu K, Inagaki M, et al. (2007) Associated and predictive factors of sleep disturbance in advanced cancer patients. Psychooncology 16: 888-894.
- Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, et al. (2009) How is sleep quality affected by the psychological and symptom distress of advanced cancer patients?. Palliat Med 23: 46-53.
- Hirst A, Sloan R (2004) Benzodiazepine and berated drugs for insomnia in palliative care. Cochrane Cochrane Database Syst Rev 4: CD003346.
- Kamell A, Smith LK (2016) Attitudes toward use of benzodiazepines among U.S. hospice clinicians: survey and review of the literature. J Palliat Med 19: 516-522.
- Chou R, Deyo R, Friendly J, Skelly A, Weimer M, et al. (2017) Systematic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 166: 480-92.
- Holbrook AM, Crowther R, Lotter A, Cheng C, King D (2000) Meta-analysis of benzodiazepine use in the treatment of insomnia. CMJ 162: 225-233.
- Gray SL, Dublin S, Yu O, Walker R, Anderson M, et al. (2016) Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. British Med J 2: 352:i90.
- Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, de Adana ES, Cano AI, Martínez-Cengotitabengoa MT, et al. (2017) Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas 101: 17-22.
- Gaudreau JD, Gagnon P (2005) Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses 64: 471-475.
- Struyk A, Gargano C, Drexel M, Stoch SA, Svetnik V, et al. (2016) Pharmacodynamic effects of suvorexant and zolpidem on EEG during sleep in healthy subjects. Eur Neuropsychopharmacol 26: 1649-1656.
- Hatahira H, Hasegawa S, Sasaoka S, Kato Y, Abe J, et al. (2018) Analysis of fall-related adverse events among older adults using the Japanese Adverse Drug Event Report (JADER). J Pharm Health Care Sci 17: 32.
- Jacobson LH, Callander GE, Hoyer D (2014) Suvorexant for the treatment of insomnia. Expert Review Clinical Pharmacology 7: 711-730.
- Sakurai T (2002) Roles of orexins in the regulation of feeding and arousal. Sleep Medicine 3: S3-9.
- Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, et al. (2010) Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem 53: 5320-5332.
- Kishi T, Matsunaga S, Iwata N (2015) Suvorexant for primary insomnia: a systematic review and meta-analysis of randomized placebo-controlled trials. PLoS ONE 10: e0136910.
- Hamasaki MY, Barberio HV, Barberio DF, Barbeiro DF, Cunha DM, et al. (2016) Neuropeptides in the brain defense against distant organ damage. J Neuroimmunol 290: 3-5.
- Walker WH, Borniger JC (2019) Molecular mechanisms of cancer-induced sleep disruption. International Journal of Molecular Sciences 20: 2780.
- Seol J, Fujii Y, Park I, Suzuki Y, Kawana F, et al. (2019) Distinct effects of orexin receptor antagonist and GABAA agonist on sleep and physical/cognitive functions after forced awakening. Proc Natl Acad Sci USA 116: 24353-8.
- Asai Y, Sano H, Miyazaki M, Iwakura M, Maeda Y, et al. (2019) Suvorexant (Belsomra® Tablets 10, 15, and 20 mg): Japanese drug-use results survey. Drugs in Rand D 19: 27-46.
- King SA, Strain JJ (1990) Benzodiazepine use by chronic pain patients. Clin J Pain 6: 143-147.
- Nielsen S, Lintzeris N, Bruno R, Campbell G, Larance B, et al. (2015) Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain Med 16: 356-366.
- Navigante AH, Castro MA, Cerchietti LC (2010) Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. J of Pain and Sym Management 39: 820-830.
- Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, et al. (2018) The role of inflammation in the pain, fatigue, sleep disturbance symptom cluster in advanced cancer. J of Pain and Symptom Management 55: 1286-1295.
- Hu S, Niu J, Zhang R, Li X, Luo M, et al. (2020) Orexin A associates with inflammation by interacting with OX1R/OX2R receptor and activating prepro-orexin in cancer tissues of gastric cancer patients. Gastroenterol Hepatol 43: 240-47.
- Thomas KS, Motivala S, Olmstead R, Irwin MR, et al. (2011) Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun 25: 53-58.
- Krasnow SM, Marks DL (2010) Neuropeptides in the pathophysiology and treatment of cachexia. Curr Opin Support Palliat Care 4: 266-271.
- Drake CL, Kalmbach DA, Cheng P, Roth T, Tran KM, et al. (2019) Can the orexin antagonist suvorexant preserve the ability to awaken to auditory stimuli while improving sleep?. J Clin Sleep Med 15: 1285-1291.
- Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A (2005) Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol 23:6712-8.
- Hatta K, Kishi Y, Wada K, Takeuchi T, Kurata A, et al. (2017) Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry 78: e970-979.
- Masuyama T, Sanui M, Yoshida N, Iizuka Y, Ogi K, et al. (2018) Suvorexant is associated with a low incidence of delirium in critically ill patients: a retrospective cohort study. Psychogeriatrics 18: 209-215.
Citation: Nishimoto T, Katayama K, Takahashi T, Kosaka H and Shimada R (2023) Efficacy and Safety of Suvorexant in a Palliative Care Unit: A Retrospective, Single-Center Study. J Hosp Palliat Med Care 5: 021.
Copyright: © 2023 Takefumi Nishimoto, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

