
Enhanced Bone Regeneration with Umbilical Cord Stem Cells: a Case Report and Histomorphometric Analysis
*Corresponding Author(s):
Dennis SmilerOral & Maxillofacial Surgeon, American Board Of Oral & Maxillofacial Surgery, Diplomate International Congress Oral Implantologist, California, United States
Tel:+1 8188490602,
Email:dennis.smiler.dds@gmail.com
Abstract
Combining bone-marrow aspirate with xenograft and allograft particulate material has been demonstrated to produce a significant quantity of new bone growth. However, securing 1 to 4 ccs of adult autogenous stem cells by means of bone-marrow aspiration is invasive, and the aspirated stem cells are typically senescent, as the procedure most often is used in aging patients. In contrast, incorporating stem cells derived from infant human umbilical cords into allograft cortico-cancellous particulate and/or bone blocks offers the promise of being a superior approach to bone regeneration. The aim of the authors was to assess the efficacy of adding umbilical cord stem cells to cancellous bone particulate allograft material to enhance repair of a significant osseous defect. Umbilical cord stem cells were mixed with particulate allograft material for use in a sinus-lift bone augmentation procedure. After three months of healing, a biopsy was taken, and the core specimen was submitted for standard histologic and histomorphometric analysis. The graft integrated well within the recipient sinus cavity. Implants were placed, and they successfully osseointegrated. Examination of the three-month post-surgery biopsy core showed the graft to be well-integrated, with 37% of the core consisting of bone, of which 100% was vital; 63% consisted of marrow. Active new bone formation was evident. In the case reported here, the use of umbilical cord stem cells in bone-graft surgery enabled successful bone regeneration, while avoiding the need for bone-marrow aspiration or autogenous donor site surgery.
Keywords
Allograft bone graft; Autogenous stem cells; Mesenchymal stem cells; Tissue engineering; Umbilical cord stem cells
ABBREVIATIONS
HSC: Hematopoietic Stem Cells
MSC: Mesenchymal Stem Cells
CD: Cluster of Differentiation
CD34+: Cluster of Differentiation protocol that identifies cell surface antigens.
CD73: Cluster of Differentiation identifies a cell?surface N?linked glycoprotein that produces extracellular adenosine
CD90: Cluster of Differentiation identifies marker of stem cells
CD105: Cluster of Differentiation identifies surface marker for receptor hematopoietic stem cells
UC: Umbilical Cord stem cells
CT: Computer Tomography
HIV: Human Immunodeficiency Virus
CMV: Cytomegalovirus
HTLV: Human T-cell lymphotropic Virus Type 1
AATB: American Association of Tissue Banks
FDA: Food and Drug Administration
INTRODUCTION
Four main elements are necessary for bone-graft success: soluble regulators, a resorbable matrix, stabilization of the matrix during healing, and cells. The most critical components are the cells, specifically osteoblasts. If osteoblasts or their precursor cells are not present at the bone-graft recipient site, they must be harvested and incorporated within the graft matrix [1] in sufficient quantity, [2] or bone will not form [3,4].
Harvested autogenous bone has long been considered the most reliable source of osteoblasts, [5,6] but in 2005 Soltan and Smiler [7] introduced a method for aspirating bone marrow to obtain adult stem cells that would differentiate to osteoblasts and could be used to enhance results from bone grafting in oral surgical applications.
Cells within the early embryo are thought to be the only single cells capable of differentiating into any cell type [8,9]. Hematopoietic Stem Cells (HSCs), for example, give rise to all the cells of the blood. Mesenchymal Stem Cells (MSCs) differentiate into bone, fat, muscle, cartilage and neurons [10,11].
In the field of bone repair and tissue regeneration, identifying a plentiful, safe and ethically acceptable source of stem cells has become an urgent need. The use of full-term human umbilical cords and placentas is an attractive option. A nearly unlimited supply of such tissue can be easily procured, and its use poses no ethical problems. Placental cells display differentiation capacity toward all three germ layers, while also having immunomodulatory effects. This raises the possibility of applying them in an allogeneic transplantation setting and may have implications in all fields of regenerative medicine and surgery [12].
Characterization of progenitor cells and stem cells is based on a variety of cell markers rather than on the cells’ morphological appearance [13]. At present, the most commonly used marker for hematopoietic cells is the cell surface marker CD34+, identified in the clinical laboratory by flow cytometry. The commonly used markers for mesenchymal stems include CD 73+, CD90+ and CD105+. Using flow cytometry, Smiler et al., identified the presence of mesenchymal stem cells, necessary for bone regeneration, in bone marrow and peripheral blood [14].
Successful transplantation of blood and marrow, both autologous and allogeneic, requires the infusion of a sufficient number of hematopoietic progenitor stem cells capable of homing into the marrow cavity and regenerating a full array of hematopoietic cell lineages in a timely fashion. Clinical studies have shown that infusion of at least 2 × 106 CD34+ cells/kg of recipient body weight results in reliable engraftment, as measured by recovery of adequate neutrophil and platelet counts approximately 14 days after the transplant [15].
Two substances that trigger the progenitors to mobilize toward the damaged tissue are growth factors and cytokines. At the same time, the progenitors also start to differentiate into the target cells. Not all progenitors are mobile; some reside near the tissue of their target differentiation [16]. When the cytokines, growth factors, and other cell-division-enhancing stimulators begin acting upon the progenitors, a higher rate of cell division is introduced [17,18].
Mesenchymal stem cells are of stromal origin [19] and may differentiate into a variety of tissues [16]. Such stem cells have been isolated from placenta, [20,21] adipose tissue, lung, marrow and blood, Wharton's jelly from the umbilical and teeth [22,23]. MSCs are attractive for clinical therapy due to their ability to differentiate, provide trophic hormone support [24] and modulate innate immune response [12]. Mesenchymal stem cells have the ability to differentiate into various cell types such as osteoblasts, chondroblasts, adipocytes, neuroectodermal cells and hepatocytes [25]. Transplanted cells lay down an initial unmineralized bone matrix (osteoid) and start the process of laying down hydroxyl apatite, the mineral component of bone in the extracellular matrix [26,27].
In order to accelerate healing of oral bone grafts, the authors have begun impregnating particulate allograft with umbilical cord stem cells. The following case report illustrates this approach to treating a patient receiving a sinus lift bone graft.
CASE REPORT
The54-year-old female patient presented with severe bone loss of the posterior region of the right maxilla. A cross-sectional view of the arch showed less than 2mm of alveolar bone between the bone crest and sinus floor (Figure 1).
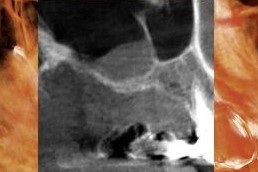 Figure 1: Cross-sectional image from the pre-operative CT scan showing the thin margin of bone between the alveolar bone crest and the sinus.
Figure 1: Cross-sectional image from the pre-operative CT scan showing the thin margin of bone between the alveolar bone crest and the sinus.
The recipient site was prepared in the following manner. First an alveolar crestal incision was made in the edentulous space with a #15 blade. Adjacent vertical relaxing incisions were created, extending into the vestibule and connecting to the lateral margins of the crestal incision. Such vertical incisions can be made one or more teeth adjacent to the edentulous area. A wide base flap ensures the necessary blood flow to the flap and periosteum.
While reflecting the flap, care was taken to preserve the periosteum without tearing it (Figure 2). A quadrilateral window osteotomy was prepared with a #6 round bur, using very light pressure to decorticate the buccal bone (Figure 3). The intact sinus membrane can be seen in figure 4.
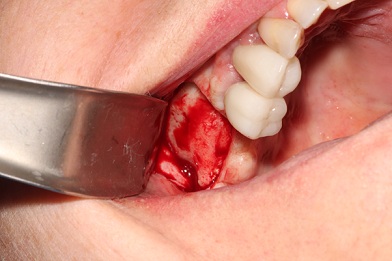
Figure 2: The mucoperiosteal flap was reflected to the level of the malar buttress.
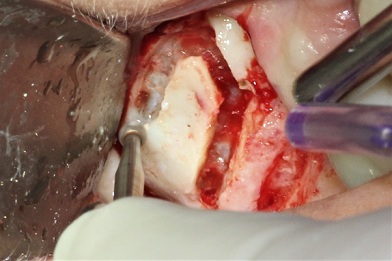
Figure 3: A quadrilateral window osteotomywas created using a #6 round bur.
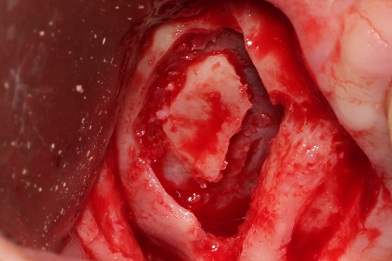 Figure 4: The intact sinus membrane can be seen prior to its elevation to expose the floor and medial wall of the sinus.
Figure 4: The intact sinus membrane can be seen prior to its elevation to expose the floor and medial wall of the sinus.
Approximately 1cc of umbilical cord stem cells (Chara Biologics, 9560 Topanga Canyon Blvd, #104, Chatsworth, CA 91311) were defrosted (Figure 5), added to 2ccs of cancellous particulate allograft bone (Impladent Ltd 86-90-188th St. Jamaica, NY) (Figure 6) and mixed to ensure thorough saturation of the bone with the Umbilical Cord Stem Cells (UC). It is estimated there are 8 million cells/cc of umbilical cord tissue matrix. Each 1cc contains approximately 20%-30% mesenchymal stem cells, thus adding over 1.6 million mesenchymal stem cells to the graft matrix. The sinus membrane was carefully lifted, from its inferior border to and including the medial wall of the sinus. The mixture of UC stem cells and cancellous allograft was inserted and loosely compacted (Figure 7). The buccal aspect of the graft was covered with a membrane processed for concentrated growth factors from patient’s peripheral blood (Figure 8). The mucoperiosteal flap was repositioned and sutured (Figure 9). Post-surgery there were no adverse reactions to the bone graft or surgery.
After 3 months of healing, a CT scan showed graft adaptation to the inferior and medial wall of the sinus (Figure 10). A core sample 4mm in diameter was obtained by passing a 4mm trephine drill through the crestal bone to the superior region of the graft. The biopsy core was fixed in 10% buffered formalin and sent for histology and quantitative histomorphometric analysis.
 Figure 5: Approximately 1cc of umbilical cord stem cells immediately after defrosting and prior to mixing with the graft material.
Figure 5: Approximately 1cc of umbilical cord stem cells immediately after defrosting and prior to mixing with the graft material.
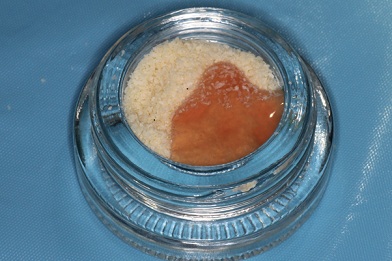
Figure 6: The UC cells were added to 2ccs of cancellous bone allograft. The two components were then mixed to achieve thorough saturation of the graft material with the UC cells.
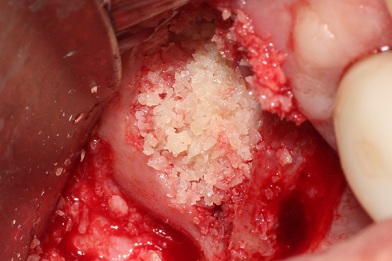 Figure 7: The combined UC cells and allograft were loosely compacted in thesinus cavity, reconstituting the buccal aspect.
Figure 7: The combined UC cells and allograft were loosely compacted in thesinus cavity, reconstituting the buccal aspect.
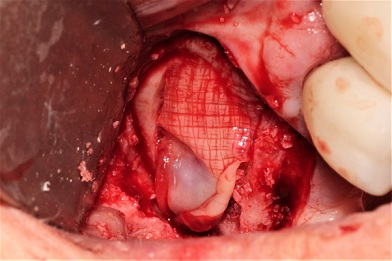 Figure 8: A membrane of concentrated growth factors was placed over the graft.
Figure 8: A membrane of concentrated growth factors was placed over the graft.
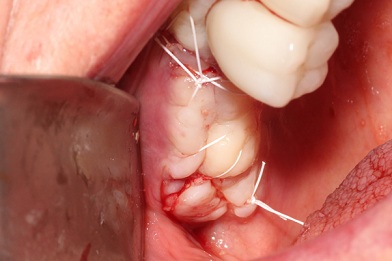 Figure 9: The mucoperiosteal flap was repositioned and sutured, tension-free.
Figure 9: The mucoperiosteal flap was repositioned and sutured, tension-free.
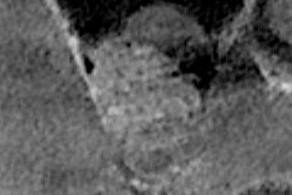 Figure 10: Cross-sectional image from the post-operative CT scan showing adaptation of the graft material within the sinus.
Figure 10: Cross-sectional image from the post-operative CT scan showing adaptation of the graft material within the sinus.
SPECIMEN PROCESSING
After dehydration with a graded series of ethanol for 9 days, the sample was infiltrated for 20 days with a light-curing embedding resin (Technovit 7200 VLC, Kulzer, Wehrheim, Germany), then embedded in Technovit 7200 VLC and polymerized by 450nm light with the temperature of the specimen not exceeding 40 degrees Centigrade. The specimen was prepared by the cutting and grinding method of Donath [28] and cut to thicknesses of 150mm on an EXAKT cutting and grinding system (EXAKT Technologies, Oklahoma City, OK), then mounted on slides. Each specimen slide was polished to a thickness of 55 microns with a series of polishing sandpaper discs from 800 to 2400 grit (EXAKT micro grinding system) followed by a final polish with 0.3-micron alumina polishing paste. Following final polishing, the specimen slides were stained using Stevenel’s blue and van Gieson’s picro fuchsin and subjected to histological evaluation by light microscopy. The specimen was evaluated using two slides to prevent sampling bias.
Microphotographs were obtained, scanned, digitized, and analyzed using a Zeiss Axiolab photomicroscope (Carl Zeiss, Jena, Germany) and Nikon Coolpix 4500 digital camera (Nikon Corp, Tokyo, Japan). The core specimen was photographed at a fixed focal point and x 25 magnification for histomorphometric evaluation. Histomorphometric measurements were completed with a Macintosh G4 computer (Apple, Cupertino, CA) and a public domain image program (NIH Images, US National Institutes of Health) along with Adobe Photoshop (Adobe, San Jose, CA). The data were exported to Microsoft Excel (Microsoft Co., Redmond, WA) for histomorphometric calculations. Histomorphometric analysis was performed and the following parameters were measured in terms of the percentage of the total core area: new bone formation, residual graft material, and marrow space.
RESULTS
The analysis showed the core to consist of bone (37%) and marrow tissue (63%). 100% of the bone was vital. No residual graft material was found (Figure 11).
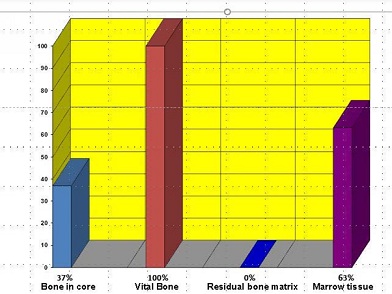 Figure 11: Analysis of the bone core taken at three months showed 37% of the core to be bone (100% vital) and 63% bone marrow. No graft material remained.
Figure 11: Analysis of the bone core taken at three months showed 37% of the core to be bone (100% vital) and 63% bone marrow. No graft material remained.
No inflammation or foreign body reaction was evident in the specimen. Overall, excellent connectivity was noted among the new trabeculae of cancellous bone, indicating active and vigorous new bone formation. Evidence that the bone formation was active also was indicated by the wide seams of new osteoid surrounding the trabeculae, which were surfaced by plump, numerous, and active osteoblasts. The staining quality of the specimen also confirmed very active new bone formation. The specific bone stain that was employed enables qualitative differentiation between the relative age of bone, the darker red being the most immature. Figures 12-19 show examples of the active new bone formation.
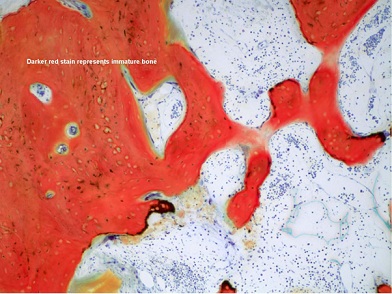 Figure 12: This low power view shows the development of new cancellous bone, indicated by the connectivity of the newly forming trabeculae.
Figure 12: This low power view shows the development of new cancellous bone, indicated by the connectivity of the newly forming trabeculae.
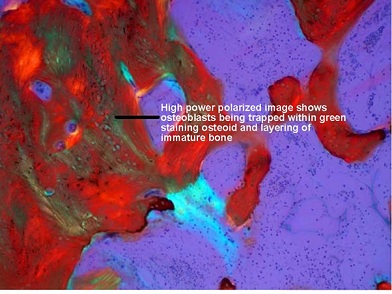
Figure 13: This polarized view emphasizes the layering of the immature bone. The bright bluish-green area in the center of the image clearly shows the early change in the bone-marrow connective tissue being induced by the presence of the osteoblasts.
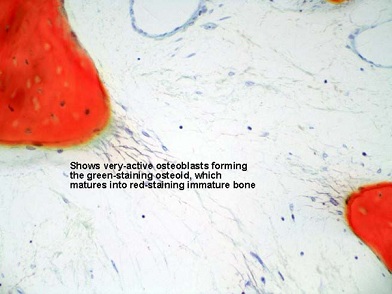
 Figures 14: These high power images show outstanding views of the dark blue osteoblasts forming the green-staining osteoid, which matures into red-staining immature bone. Absolutely no inflammation or other abnormality is evident.
Figures 14: These high power images show outstanding views of the dark blue osteoblasts forming the green-staining osteoid, which matures into red-staining immature bone. Absolutely no inflammation or other abnormality is evident.
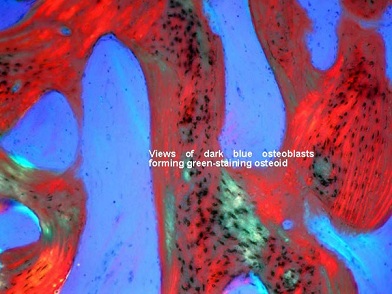 Figures 15: These high power images show outstanding views of the dark blue osteoblasts forming the green-staining osteoid, which matures into red-staining immature bone. Absolutely no inflammation or other abnormality is evident.
Figures 15: These high power images show outstanding views of the dark blue osteoblasts forming the green-staining osteoid, which matures into red-staining immature bone. Absolutely no inflammation or other abnormality is evident.
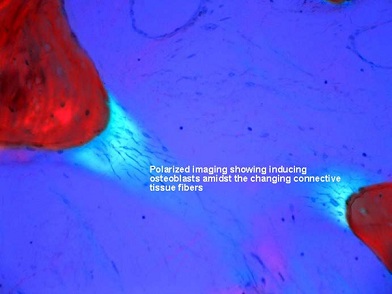
 Figures 16: This matched pair of images shows normal imaging adjacent to polarized imaging that emphasizes the induction of the connective tissue in the delicate tissue, which will become bone marrow, and then bone. The inducing osteoblasts can be seen amidst the changing connective tissue fibers.
Figures 16: This matched pair of images shows normal imaging adjacent to polarized imaging that emphasizes the induction of the connective tissue in the delicate tissue, which will become bone marrow, and then bone. The inducing osteoblasts can be seen amidst the changing connective tissue fibers.
 Figures 17: This matched pair of images shows normal imaging adjacent to polarized imaging that emphasizes the induction of the connective tissue in the delicate tissue, which will become bone marrow, and then bone. The inducing osteoblasts can be seen amidst the changing connective tissue fibers.
Figures 17: This matched pair of images shows normal imaging adjacent to polarized imaging that emphasizes the induction of the connective tissue in the delicate tissue, which will become bone marrow, and then bone. The inducing osteoblasts can be seen amidst the changing connective tissue fibers.
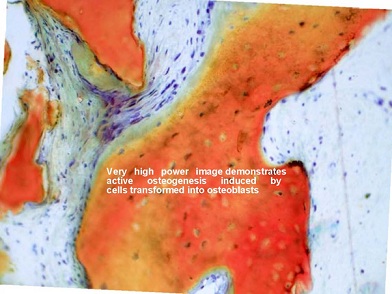 Figure 18: Very high-power images further demonstrate the active osteogenesis instigated by the cells that have become transformed into osteoblasts.
Figure 18: Very high-power images further demonstrate the active osteogenesis instigated by the cells that have become transformed into osteoblasts. 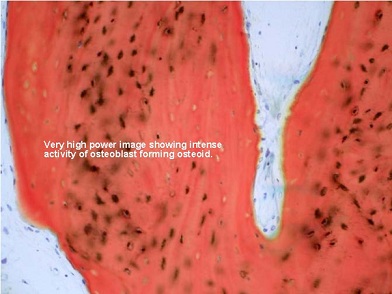
Figure 19: Very high-power images further demonstrate the active osteogenes is instigated by the cells that have become transformed into osteoblasts.
DISCUSSION
Umbilical cord (UC) blood was first reported as a source of hematopoietic stem and progenitor cells in 1974. In 1988, the first transplantation of cryopreserved UC blood to an infant with an inherited bone-marrow disease took place in France. Banking of cord blood began around 1992, but for years UC tissue continued to be regarded as mere medical waste. It was not until the last decade that recognition of the significant therapeutic value of UC tissue dawned. Such tissue contains high concentrations of MSCs as well as an abundance of growth factors and extracellular matrix [29].
UC-derived MSCs (UC-MSCs) may have many advantages over the traditional gold standard of bone-marrow-derived MSCs. These advantages include higher proliferative potential, better resistance to senescence, wider differentiation capacities, stronger anti-inflammatory and paracrine-signaling activity, as well as more potent immunomodulatory effects.
UC-MSCs can be processed as soon as birth tissue is collected. Ideally, the tissue should be obtained from young healthy donor mothers who have chosen to undergo elective Cesarean section. This helps to assure the tissue sterility. Donor screening is crucial to ensuring the quality of the tissue and maximizing its therapeutic potential. The product used by the present authors was subjected to some of the most stringent screening in the industry. This includes documentation of the mother's medical history, family history, travel history, occupational history, infectious disease and toxic exposures and sexual history, as well as her sexual partner's history and the baby's prenatal history. When birth tissues are obtained, they are screened for HIV, syphilis, hepatitis B, hepatitis C, CMV, HTLV and other infectious diseases. After processing and before the product is released for clinical use, sterility (all fungi, mold, yeast, bacteria) and endotoxin testing also are performed in accordance with AATB and FDA standards.
Most products derived from UC tissue are composed of cells from the cord only, but CharaCore® is unique in the comprehensiveness of its contents. This product contains cells from the entire umbilical cord, which includes the UC tissue, the amniotic membrane and some UC blood. This results in a variety of cell types being included in the product. MSCs are guaranteed to constitute at least 20% of the total cell population, but HSCs are also present, along with endothelial and epithelial progenitor cells and other immature immune cell types. These cells likely work synergistically, evidenced by the fact that both MSCs and HSCs promote angiogenesis, and MSCs are well known for enhancing the activities and engraftment of HSCs.
In CharaCore®, all these cells are suspended in the serum of the cord blood, which provides additional and crucial growth factors and cytokines. The cells are thus maintained in a solution as close to their natural niche environment as possible. When cells are maintained in their niche, they function optimally. When they are taken out of their niche, they often behave in a completely different manner.
CONCLUSION
The technique described here of combining allograft particulate cancellous bone with umbilical cord stem cells broadens the options available to dental clinicians seeking to augment patients’ maxillary or mandibular bone as part of an overall strategy for oral rehabilitation. Further study of the use of umbilical cord stem cells in a variety of bone graft surgical procedures is recommended.
REFERENCES
- Smiler DG (1998) Bone grafting: materials and modes of action. Pract Periodontics Aesthet Dent 8: 413-416.
- Smiler DG, Soltan M (2006) The bone-grafting decision tree: a systematic methodology for achieving new bone. Implant Dent 15: 122-128.
- McCain JP, Marx RE (1978) A retrospective study of the donor site in bone grafts from the ilium. Read before the AAOMS 60th Annual Meeting.
- Burwell RG (1964) Studies in the transplantation of bone. The fresh composite homograft autograft of cancellous bone. J Bone Joint Surg 46: 110-140.
- Burwell RG (1966) Studies in the transplantation of bone. 8. Treated composite homograft-autografts of cancellous bone: an analysis of inductive mechanisms in bone transplantation. J Bone Joint Surg 48: 532-566.
- Lindholm TS, Nilsson OS (1982) Extraskeletal and intraskeletal new bone formation induced by demineralized bone matrix combined with marrow cells. Clin Orthop Relat Res 171: 251-255.
- Soltan M, Smiler DG, Gailani F (2005) A new “platinum” standard for bone grafting: autogenous stem cells. Implant Dent 14: 322-325.
- ScienceDaily (2012) First-of-its-kind stem cell study re-grows healthy heart muscle in heart attack patients. ScienceDaily, USA.
- Fraser CC, Szilvassy SJ, Eaves CJ, Humphries RK (1992) Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci USA 89: 1968-1972.
- Fukuchi Y, Nakajima H, Sugiyama D, Hirose I Kitamura T, et al. (2004) Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 22: 649-658.
- Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, et al. (2004) Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32: 370-373.
- Evangelista M, Concini M, Parolini O (2008) Placenta-derived stem cells: new hope for cell therapy? Cytotechnology 58: 33-42.
- Morgan JE, Partridge TA (2003) Muscle satellite cells. Int J Biochem Cell Biol 35: 1151-1156.
- Smiler D, Soltan M, Albitar M (2008) Toward the identification of mesenchymal stem cells in bone marrow and peripheral blood for bone regeneration. Implant Dent 17: 236-247.
- To LB, Haylock DN, Simmons PJ, Juttner CA (1997) The biology and clinical uses of blood stem cells. Blood 89: 2233-2258.
- Mlsna, Lucas J (2010) Stem Cell Based Treatments and Novel Considerations for Conscience Clause Legislation. Indiana Health Law Review. United States: Indiana University Robert H. McKinney School of Law 8: 471-496.
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1: 2315-2319.
- Yates DS, Moore DS, Starnes DS (2003) The Practice of Statistics (2ndedn.). New York: Freeman. ISBN 978-0-7167-4773-4.
- Farhi DC (2008) Pathology of bone marrow and blood cells (2ndedn). Lippincott Williams & Wilkins, USA.
- Clark DA, Chaput A, Tutton D (1986) Active suppression of host-vs-graft reaction in pregnant mice. VII. Spontaneous abortion of allogeneic CBA/J x DBA/2 fetuses in the uterus of CBA/J mice correlates with deficient non-T suppressor cell activity. J Immunol 136: 1668-1675.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P et al. (2015) Tissue-based map of the human proteome. Science 347: 1260419.
- Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells 25: 2896-2902.
- Shi S, Bartold PM, Miura M, Seo BM, Robey PG, et al. (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 8: 191-199.
- Steinberg W (1952) Trophic Vs. Tropic. JAMA 149: 82.
- Bai X, Alt E (2010) Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun 401: 321-326.
- Smiler D, Soltan M (2007) A histomophogenic analysis of bone grafts augmented with adult stem cells. Implant Dent 16: 42-47.
- Smiler D (1996) Small-segment symphysis graft: augmentation of the maxillary anterior ridge. Pract Periodontics Aesthet Dent 8: 479-481.
- Rohrer MD, Schubert CC (1992) The cutting-grinding technique for histological preparation of undecalcified bone and bone-anchored implants: Improvement in instrumentation and procedures. Oral Surg Oral Med Oral Pathol Endod 74: 73-78.
- Arutyunyan I, Fatkhudinov T, Sukikh G (2018) Umbilical cord tissue cryopreservation: a short review. Stem Cell Res Ther 9: 236.
Citation: Smiler D, Rosen D, Kong J, Prasad S, Prasad HS (2020) Enhanced Bone Regeneration with Umbilical Cord Stem Cells: a Case Report and Histomorphometric Analysis. J Stem Cell Res Dev Ther 6: 054.
Copyright: © 2020 Dennis Smiler, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

