
Epidemiology and Public Health Importance of Toxoplasma Gondii in Ethiopia: An Overview
*Corresponding Author(s):
Yehualashet Behailu WoldesenbetHawassa University School Of Postgraduate Study, Faculty Of Veterinary Medicine Department Of Veterinary Epidemiology, Hawassa, Sidama, Ethiopia
Tel:+251-975711302,
Email:yehualashet.behailu@gmail.com
Abstract
Toxoplasma gondii is one of the most widely prevalent cyst forming apicomplexan parasites. Its causative agent is toxoplasmosis, facultatively heteroxenous a zoonotic protozoan parasite that infects virtually all warm-blooded animals, including wildlife, domestic animals and humans. Epidemiologically T. gondii infection and transmission are multifaceted, involving the three developmental stages (tachyzoite, bradyzoite, and sporozoite) of the parasites during sexual and asexual life cycle. Ingesting infectious oocysts from the environment or ingesting tissue cysts or tachyzoites which are contained in meat or primary offal (viscera) of many different animals. Horizontal and vertically transmission is possible. Felids are a crucial role in the epidemiology of toxoplasmosis issue because they are the only hosts that can excrete environmentally resistant oocysts and acquire infections via the ingestion of sporulated oocysts. Human beings and other warm-blooded animals, which serve as intermediate hosts, are infected primarily via contaminated water, vegetables and fruits, Consumption of raw or undercooked meat and congenital transmission from the mother to fetus via the placenta. The parasite has a significant impact on Public Health and animal production, reproductive loss and causes mortality in neonatal. Most cases of exposure result in subclinical infection with no clinical signs. Diagnosis comprises direct, immunodiagnostic and molecular techniques are crucial for the surveillance, prevention and control. In clinical cases intensive supportive treatment may be necessary with combinations of certain antibiotics. Prevention and control is centered on avoidance of contact with sources of infection, such as cats, consumption of contaminated raw or undercooked meat, contaminated environment, personal hygiene and regular hand washing. Collaborative and focused control strategies should be implemented for this serious disease.
Keywords
Bradyzoite; Control and Prevention; Epidemiology; Public health; Sporozoite; Toxoplasma gondii; Tachyzoite
Introduction
Parasitic infections are amongst the most common infections throughout the world [1]. The diseases are common in developing countries because of the poor sanitation practices, inadequate food safety laws, weak regulatory systems and lack of financial resources [2] parasitic diseases, it includes protozoa, helminths, arthropods, they are ectoparasites and endoparasites. Among these parasitic diseases protozoa is most important [3]. Toxoplasma is now recognized as the most common protozoan parasite globally, with the widest range of hosts spread over 200 species of birds, reptiles and mammals, including humans [4]. Toxoplasma gondii, the causal agent of toxoplasmosis, is an important water and food borne protozoan parasite ubiquitous throughout the world [5]. It belongs to the phylum apicomplexa along with other well-known member’s like-Plasmodium, Sarcosystis and Neospora [6]. The Apicomplexa are a parasitic phylum comprised of obligate intracellular eukaryotes, which can only replicate inside the cells of their hosts [7-9].
Felid the only known definitive hosts. There are three known invasive stages in the complex heteroxenous life cycle of T. gondii: tachyzoites, bradyzoites and sporozoites inside the oocysts [10-11]. It can undergo asexual reproduction in tissues of Felidae acting as intermediate hosts. First, tachyzoites have active multiplication in tissues, associated to rapid invasion causing harmful effects and present a special tropism to central nervous system and striated muscle, in which they remain latent confined in a cyst as bradyzoites, leading to a long-term chronic infection until another definitive host ingests the tissue. Then, released bradyzoites penetrate the epithelial cells of small intestine, giving rise to schizonts that will form gamonts and finally oocysts. Felids excrete oocysts in their faeces, during a limited time lapse, contaminating soil and water [12].
Toxoplasmosis is a zoonotic disease and its notable prevalence in important meat-producing animal species such as pigs, poultry and goats is a major public health concern [13].Toxoplasmosis imposes a substantial disease burden in humans all across the world, with an estimated third of the global population infected and seroprevalence figures above 60% in some parts of South America, Africa and South-East Asia [14]. In pregnant women exposed for the first time, tachyzoites can therefore reach the fetus [15]. Acute toxoplasmosis causes cold symptoms or, in rare cases, prolonged fever, fatigue, retinochoroiditis, painless cervical lymphadenopathy and seizures [16]. In chronic toxoplasmosis, bradyzoite cysts are found in soft tissues such as the lungs and brain [17] Immunocompromised with latent toxoplasmosis can suffer from meningoencephalitis and mental complications [18].
Major routes transmission are different commonly occurs through the ingestion of food and water contaminated with infectious oocysts shed from cats or by ingesting undercooked meat containing the viable tissue cysts [19]. Diagnosis is performed serological tests. Such as histology, immunohistochemistry, PCR, ELISA and IFAT widely used to detect T. gondii However, several measures can be adopted to prevent or minimize the risks of contamination. These include hygienic methods such as avoiding contact with or washing hands after contact with cats and their litters, wearing gloves while gardening and washing raw fruits and vegetables thoroughly before consumption. Toxoplasma tissue cysts in meat can be killed by cooking at 67°C [20].
A review particular contemplation, in my rumored, toxoplasmosis is significant but often neglected threat as causing huge economic loss and public health problem in developing countries. Therefore considering the epidemiology and public health importance of the disease but giving less attention especially in our counter, to combat, a review protozoan Parasitic was accompanied with objective of
- To review the epidemiology and public health importance of Toxoplasma gondii
- To alert and provide information on the effective control and preventive measures taken against the disease
Review
- Description of toxoplasma gondii
Toxoplasma gondii is a cosmopolitan parasite with a variable frequency worldwide. It is estimated that T. gondii infects one third of world population, this protozoan is one of the most common parasites, which infects human and other warm-blooded animals.
Taxonomy
Toxoplasma gondii is a protozoan parasite of the phylum Apicomplexa, taxonomic group of endoparasites of animals characterized by the presence of an apical complex in its cellular structure, with more than 6000 members. The infections involving apicomplexan parasites represent a huge burden on public and animal health worldwide [21]. The parasite Domain: Eukaryota, Kingdom: Alveolata, Phylum: Apicomplexa, Class: Coccidia, Subclass: Eucoccidiorida, Order: Eimeriorina, Family: Sarcocystidae, Genus: Toxoplasma, Species: Toxoplasma gondii
Etiology
Toxoplasma gondii, the etiologic agent of toxoplasmosis, is an apicomplexan obligate intracellular parasite of major medical and veterinary importance.
Historical Aspect
Toxoplasma gondii was first discovered in the spleen of the North African rodent, the gondii (Ctenodactylus gundi). It has been more than a century since the discovery of Toxoplasma at the Pasteur Institute in Tunis. In 1908, while performing research on leishmaniasis using a hamster-like rodent called Ctenodactylus gundi, Nicolle and Manceaux discovered a new protozoan in the tissues of two dead animals. Initially naming this parasite Leishmania gondii, Nicolle and Manceaux later recognized it as a separate genus renaming it Toxoplasma gondii [22].
The parasite was also seen by Splendore, (1908) in tissues of a rabbit in Brazil. Congenital toxoplasmosis was first reported in 1937 [23-24] As early as 1937 Sabin and Olitsky, two virologists working with guinea pig brains, noticed that Toxoplasma multiplication was only possible inside a living cell, describing it as an obligate intracellular parasite for the first time. They also reported the possibility that ‘one method of natural dissemination may be by means of the eating of toxoplasma-contaminated tissues’ after mice fed on Toxoplasma-infected mice also became infected. Interestingly, they noted the serum of recovering rhesus monkeys contained “neutralizing” or “protective” antibodies [25].
- Life cycle of Toxoplasma gondii
There are three known invasive stages in the complex heteroxenous life cycle of T. gondii: tachyzoites, bradyzoites contained within the tissue cysts, and sporozoites inside the oocysts
Tachyzoites
Tachyzoites are crescent shaped cells of approximately 2 x 6 µm, capable of invading almost all types of nucleated cells, developing inside a structure called parasitophorous vacuole Tachyzoite, derived from the Greek word tachos, meaning speed, to describe this rapidly dividing stage. Tachyzoite stage relates to the form of fast multiplication of T. gondii during the acute phase of infection [26].This was also the stage that Nicolle and Manceaux, found in the gundi rodent in 1909 [27].
Bradyzoites and tissue cyst
Bradyzoites are the slow multiplication stage of T. gondii present during the chronic phase of infection. Bradyzoites are localized in tissue cysts. It located in various host cells but mainly in neurons and in heart and muscle cells.Tissue cysts of T.gondii are found in meat of any warm-blooded animal. They can persist for the life of the host. Although tissue cysts grow in the visceral organs such as the lungs, liver, and kidneys, they are mostly found in the neural, central nervous system and muscular tissues which include the eyes and skeletal/cardiac muscles [28-30].
Oocyst
Sporozoites contained within the sporulated oocysts of T.gondii are the result of asexual reproduction in cats or other felids [31]. Toxoplasma gondii oocysts are excreted to the environment in faeces by the definitive host (only members of the Felidae family) [32] Oocysts in cat feces are unsporulated and non-infective. They mature in the environment and after sporulation, they contain 2 sporocysts with 4 sporozoites in each of them.They are disseminated by rain and surface water, which leads to contamination of the environment. How often cats shed oocysts in the environment is unknown but cats can shed millions of oocysts after ingesting only one bradyzoite. The sporulated oocysts of T.gondii are resistant to harsh climatic circumstances [33].
Life cycle in the intermediate and definitive hosts
The life cycle of T.gondii is complex. It comprises a phase of sexual reproduction in definitive hosts (Felidae), especially cats. It also comprises a phase of asexual reproduction that occurs in intermediate hosts (birds and mammals) including humans [34] as well as in definitive hosts.
Life cycle in intermediate hosts
The asexual cycle after ingestion of oocysts by the intermediate hosts (birds and mammals), the sporozoites released from the oocysts and penetrate into the intestinal epithelial cells, where they transform into tachyzoites and move to the lamina [35] propria where they multiply asexually by endodyogeny in a variety of nucleated cells until dividing into tachyzoite forms. The tachyzoites are able to infect any nucleated cell type and disseminate throughout the body via infected blood cells. After a few days, some of these tachyzoites transform into bradyzoites, gathered in cysts mainly found in nerve and muscle cells. The cysts of T. gondii could persist throughout the host life. However, tissue cysts could break down periodically: bradyzoites transform to tachyzoites and reinvade host cells to form new tissue cysts
Bradyzoites continue multiplying slowly by endodyogeny inside the tissue cyst. The bradyzoite-induced cycle in the intermediate host is similar to that of the oocyst-induced cycle but bradyzoites are apparently less infective than sporozoites [36].
Life cycle in definitive hosts
Enteroepithelial sexual stage of Toxoplasma gondii carries out the sexual portion of its life cycle exclusively in the small intestine of the definitive host after the ingestion of tissue cysts by cats, the tissue cyst wall is digested by proteolytic enzymes in the stomach and small intestine. The released bradyzoites (haploid) penetrate the epithelial cells of the small intestine and initiate the asexual development of numerous generations of T. gondii
They firstly undergo a limited number of asexual multiplications (schizogony). Merozoites resulting from these first steps of asexual multiplications in enterocytes produce male or female gametocytes that will mature into gametes. The fertilization between male and female gametes leads to the production of unsporulated oocysts or non-infecting oocysts. These oocysts are excreted in the feces of Felidae and become sporulated in the environment [37] In the environment sporogony occurs in the within 1 to 5 days given suitable conditions of aeration, humidity and temperature. Sporogony involves meiosis (postzygotic) and sporulation, ultimately producing two sets of four haploid sporozoites, contained within a second set of walled structures, called sporocysts. The oocysts are very resistant (Figure 1) and infective for both intermediate and definitive hosts. Cats can excrete several millions of oocysts that disseminate in the environment [38].
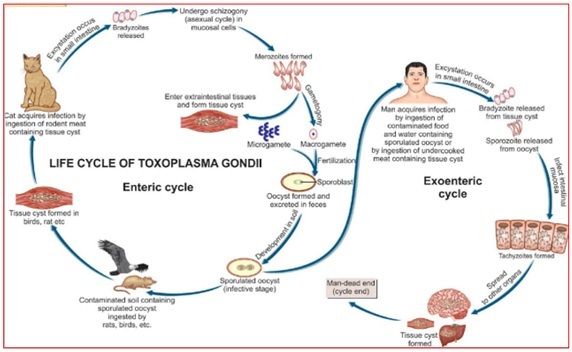 Figure 1: Life cycle of Toxoplasma Gondii
Figure 1: Life cycle of Toxoplasma Gondii
- Mode of transmission
Horizontal transmission
T.gondii may be transmitted from definitive to intermediate hosts, from intermediate to definitive hosts, as well as between definitive and between intermediate hosts. Consuming tissue cysts by eating raw meat, undercooked or insufficiently frozen (lamb, pork, cow, beef, chicken, horse,), ingestion of oocysts present in an environment contaminated by cat feces: plants (fruit, vegetable from the garden...), water, soil (gardening or farming activities), and direct contamination by cat by handling dropping litter in the absence of a proper hygiene are the main sources. Contamination through blood transfusion or organ transplant is quite possible although much infrequent [39].
Vertical transmission
Congenital transmission of T. gondii is a result of transplacental transmission of tachyzoites [40]. Parasitemia during pregnancy may result in placentitis, tachyzoites bypassing the placental blood barrier and invasion of the foetal organs compromising the developmental process [41]. Congenital infection found to occur in many species of animals, particularly sheep, goats, and rodents. Some strains of mice with infected mice producing congenitally infected. Women are infected during the first two months of gestation [42, 43]. The risk of symptomatic congenital toxoplasmosis and the severity of the disease are inversely related to the week of gestation in which transmission occurs. Thus, the highest frequency of severe abnormalities at birth is seen in children whose mother acquired a primary infection with T. gondii between the 10th and 24th week of gestation as shown in (Figure 2)
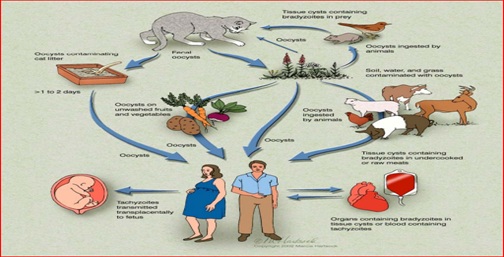 Figure 2: Mode of Transmission of Toxoplasma Gondii
Figure 2: Mode of Transmission of Toxoplasma Gondii
- Epidemiology
Toxoplasma gondii infection in animals and humans is widespread all over the world [44-47]. It is considered that approximately one-third of human population is infected with T. gondii worldwide although figures vary notably between countries (from 10 to 80%) [48].The seroprevalence in felids is a crucial issue because they are the only hosts that can excrete the environmentally resistant oocysts and can directly transmit the parasite to humans and livestock. T. gondii infection varies widely according to the age, lifestyle (stray, wild, or domestic), breed and country ranging between 0 to 100% Seroepidemiological studies in humans as well as a broad range of animal species which provided evidence for a wide distribution and high prevalence of T. gondii in many areas of the world [49].
Source of infection and occurrence
Commonly acquired by the ingestion of tissue cysts that contain bradyzoites or by the ingestion of oocysts containing sporozoites. Sporozoites are the product of a sexual cycle in cat intestines. The sources of infection could vary greatly between different ethnic groups and different geographical locations. Mainly the presence of felids in their environment, the climate conditions (favoring sporulation and survival of oocysts in the environment), susceptibility to toxoplasma infection (some species may be more resistant) or the diet and feeding behavior of the host species.
However, while consumption of raw or undercooked meat was consistently identified as a risk factor [50]. Outbreaks of acute toxoplasmosis are usually linked to an occasional point source of infection and thus, do not necessarily reflect the major, epidemiologically important sources [51].
Host range and susceptibility
T.gondii is ubiquitous parasite that occurs in most areas of the world. It is capable of infecting an unusually wide range of hosts and many different host cells [51] The infection has been described for more than 350 host species, mammals and birds, with the vast majority of them living in a wild environment including human being. Felid hosts, the only known definitive hosts for T. gondii and toxoplasmosis is a zoonotic disease that major public health concern.Cattle and horses are considered highly resistant to clinical toxoplasmosis whereas sheep and goats are highly susceptible and pigs remain at an intermediate degree of susceptibility.
Distribution of toxoplasmosis in Ethiopia
In Ethiopia the risk factor associated with seropositivity to toxoplasma infection were consumption of raw or undercooked meat and presence of cat in the households [52,53]. There are no reports of clinical toxoplasmosis in animals. Serological surveys indicate a high prevalence of T. gondii antibodies in sheep and goats. Recently [54] reported 52.8% in Small Ruminants from Yabello, Borana Zone and reported 74. 9% from central and southern regions of Ethiopia.
The studies have found a seroprevalence of 85.4% of feral cats sampled in Ethiopia [55]. The overall prevalence recorded in sheep in Ethiopia and other African countries is 54.7% [56-60]. Ethiopia reported prevalence of toxoplasmosis in the general population ranging from 20.2% to 97.7% and the seroprevalence study of human T. gondii infection of workers at Addis Ababa abattoir, reported a prevalence of 96.77% [60-65] Relative prevalence of toxoplasmosis in different species indicated by is Ovine- 55.91% , Caprine- 53 %, Bovine- 12.68%, Swine-11.53%, Equine- 5.76%, Camel- 2.59% as shown in (Table 1).
|
Author |
Year of study |
Host |
Location |
Prevalence (%) |
|
Zewdu et al. |
2010-2011 |
Goat |
Central Ethiopia; |
19.74 |
|
Dechassa et al., |
2016 |
Sheep, goats |
Southwestern Ethiopia |
57.60% |
|
Negash et al., |
2004 |
Sheep, goats |
Nazareth |
57.60% |
|
Negash et al., |
1999–2000 |
Sheep, goats |
Nazareth |
43.10 |
|
Dubey et al., |
2011 |
Cats |
Addis Ababa |
22.22 |
|
Zewdu et al., |
2013 |
caprine |
Central Ethiopia |
19.7.4 |
|
Tilahun et al., |
2012 |
Chicken |
Central Ethiopia; |
38.40 |
|
Tiao |
2012 |
Cat |
Central Ethiopia |
85.42 |
|
Gebremedhin et al., |
2012–2013 |
camels |
Central Ethiopia |
47.85 |
|
Demissie and Tilahun |
2000–2001 |
Sheep, goat |
Central Ethiopia |
33.97 |
|
Teshale et al., |
2005-2006 |
Sheep |
Central and Southern Ethiopia |
74.88 |
|
Kula et al., |
2021 |
Sheep, goat |
Yabello, Borana Zone |
52.8 |
|
Yohannes et al., |
2012-2013 |
Human |
Northern Ethiopia |
7.83 |
|
Shibre et al.,
|
2005 |
Human |
Central Ethiopia |
88.89 |
|
Shimelis et al., |
2007 |
Human |
Addis Ababa |
93.3 |
|
Zemene et al.,
|
2011 |
Human |
Southeast Ethiopia |
81.09 |
|
Aleme et al., |
2011–2012 |
Human |
Central Ethiopia |
94.00 |
Table 1: Prevalence of animal and human toxoplasmosis in different part of Ethiopia.
- Public health and socio-economic importance
Zoonotic Importance
Toxoplasma gondii is a zoonotic protozoan parasite that infects virtually all warm-blooded animals, including wildlife, domestic animals, and humans [59] .Disease in humans caused by T. gondii was first recognized in the late 1930s.The rate of infection in humans and other animal hosts has been reported differently in various parts of the world. Toxoplasma infection in humans, especially in people with defective immune system, pregnant women, HIV/AIDS patients, children and those with underlying disease could entail serious damage [66-68] Toxoplasmosis ranks high on the list of diseases that lead to the death of patients with AIDS Infection of pregnant women may result either in abortion or congenital infection of the fetus and also resulting hydrocephalus, intracranial calcification and retinochoroiditis.
Economic Importance
Its effects are particularly important in sheep and goats because it causes abortions and disease in newborns, resulting in serious economic losses The economic impact of diseases has different aspects that need to be taken into account. Direct costs of a disease include not only losses, but also costs for the treatment of animals and costs for disease prevention. The first aspect is ‘the value of the loss in expected output and/or of resource wastage due to the disease. T. gondii is considered a major cause of reproductive losses in the small ruminant industry worldwide and infections in small ruminants may play a major role in the transmission of the parasite to humans [63-64].
In sheep, lambs, wool, milk and meat represent the main output of a flock. Primary infection of sheep with T. gondii, there is a high probability of abortion (Dubey, 2009). Congenitally infected lambs lack muscular coordination, they are physically weak, and they are unable to feed themselves.The socio-economic impact of toxoplasmosis in humans suffering and the cost care of sick children, especially those with mental retardation and blindness are enormous. The clinical disease usually occurs sporadically and has low levels of incidence [65].
- Clinical Manifestations
There are four forms of toxoplasmosis; subclinical, sub-acute, acute and chronic (latent). Most cases of exposure result in subclinical infection with no clinical signs.
In Animal
Cats and other felids
The vast majority of infections in domesticated cats are asymptomatic. Most cases of toxoplasmosis seem to occur in young or immunocompromised cats, acute toxoplasmosis include lethargy, persistent fever and anorexia. Many cats develop respiratory signs, including dyspnea, acute abdominal condition such as hepatitis (e.g., hepatomegaly, abdominal tenderness, diarrhea and occasional vomiting. The specific signs depend on the site(s) affected in the brain or spinal cord [66-67].
Sheep, goats and cattle
T.gondii usually infects adult sheep and goats without clinical signs; however, infections acquired during pregnancy can cause abortions, stillbirths, and mummification or resorption of the fetus. Fetuses infected early in gestation are affected the most severely and deaths are common. Congenitally infected lambs may be in coordinated, weak and unable to nurse and often die [68]. Cattle and horses are considered highly resistant to clinical toxoplasmosis and pigs remain at an intermediate degree of susceptibility.
Other intermediate hosts
Outbreaks of toxoplasmosis, with abortions, stillbirths, mummified fetuses, neonatal mortality and/or generalized illnesses. In other mainly characterized by reproductive losses, illnesses and deaths sometimes occur in older animals, toxoplasmosis in dogs can cause reproductive losses, sometimes with stillbirths and apparently healthy pups in the same litter.
In Human
In Human infection by T. gondii is asymptomatic in over 80% of cases or causes a benign illness in approximately 20% of cases. Pregnant hosts that acquire the infection, during a chronic reactivated infection, the congenital infection manifests mainly in the CNS (of the foetus in this case).The incubation period is estimated to be 5-23 days.
In humans, common signs reported in congenitally infected infants are hydrocephalus or microcephalus, cerebral calcifications, retinochoroiditis and long-term disabling sequelae, retinochoroiditis or neurological involvements could also appear later in life Localized toxoplasmosis the most common location is the brain; the clinical characteristic is that of a cerebral abscess and encephalitis. The second most frequent location is eye. The patient complains of a declined visual acuity and eye redness. Pulmonary toxoplasmosis leads to a feverish interstitial pneumonia . In immunocompromised patient’s serious illness, constantly threatening without treatment. Severe manifestations such as encephalitis, sepsis syndrome/shock, myocarditis, or hepatitis may occur, but are very rare in immunocompetent humans [69-70].
Pathogenesis
The virulence factors of the parasite and immune defenses of the host might drive the species-dependent susceptibility to toxoplasmosis [70-71] Obviously, the immune status of the host also influences the development of the disease. It considered the most frequent opportunistic pathogen in AIDS patients and there are numerous reports of infections after immunosuppressive [72-73]. Toxoplasma infection, with the infection usually being in apparent or producing only transient mild symptoms during the acute phase, although the host remains chronically infected for its lifetime [74].
Toxoplasma gondii infection outcome varies depending on the genetic background and immune status of the host, as well as on the parasite genotype. The resistance or susceptibility to infection seems to differ depending on the host species and even the subspecies, as has been demonstrated in the case of rodents. T. gondii infection influencing a range of human behaviors and personality disorders were evaluated psychiatric patients the primary diagnoses like alzheimer's, schizophrenia, major depression, schizoaffective, or bipolar [75-80].
- Detection Methods
The diagnosis of T. gondii infection is crucial for the surveillance, prevention and control of toxoplasmosis [81].
Microscopic diagnosis
These techniques imply the detection of the parasite based on light microscopy. Despite having been traditionally used, they have a low sensitivity and require skilled personnel to obtain reliable detection results [82]. Oocysts could be identified on faecal (felids), water, soil, or food (i.e., susceptible to T. gondii presence like vegetables or fruit) samples, and even in aerosols, after filtration and centrifugation processes [83,84].
Bioassay: Cats are the most sensitive bioassay model for the detection of T. gondii in meat because an animal can be fed with much larger volumes of tissues (500 g or more) and can excrete millions of oocysts after ingesting only one bradyzoite [85-87].
Molecular methods -DNA detection
PCR-based diagnosis methods cover the inherent limitations of traditional diagnostic methods, being much more sensitive and specific. Nested-PCR targeting multicopy genes, usually used for the detection of T. gondii in biological samples, where in some cases the parasite burden is extremely low (e.g., blood samples) [79]. PCR amplification is very important for detection of T. gondii DNA in body fluids and tissues and enables an [88] early detection of T. gondii DNA in brain tissue, cerebrospinal fluid (CSF), Vitreous and aqueous fluids, bronchoalveolar lavage (BAL) fluid and blood [80].
Serological assays
The serologic tests for demonstration of specific antibody to T. gondii are the initial and primary method of diagnosis. Based on specific antibodies detection (indirect diagnosis) to determine the time point of infection (recent/chronic), a valuable alternative is the detection of circulating antigens in serum (direct diagnosis) in ELISA has been designed .A combination of serologic tests is required to measure different antibodies that possess unique patterns of rise and fall with time after infection [89]. Toxoplasma l Profile (TSP), ELISA and AC/HS tests are used to determine infection acquired in the recent or more distant past [90-93].
Treatment
Intensive supportive treatment may be necessary in animals with disseminated disease. Clinical cases are treated with [94] antibiotics. Only certain drugs, such as clindamycin and azithromycin used alone or in various combinations, are effective. The most effective treatment of toxoplasmosis is a combination of the oral antibiotic drugs pyrimethamine and sulfadiazine plus the B vitamin folinic acid. Corticosteroids may be administered concurrently in ocular disease to reduce inflammation. While antibiotics can suppress actively dividing parasites, they cannot destroy tissue cysts and are unlikely to completely eliminate T. gondii from the body [95-98].
Prevention and control strategies
To prevent food-borne horizontal transmission of T. gondii, meat and other edible parts of animals should not be consumed raw or undercooked, i.e. they should be cooked thoroughly (67°C) before consumption. Deep-freezing meat (−12°C or lower) before cooking can reduce the risk of infection. In addition, meat should not be tasted during seasoning or cooking [84] The main strategies for controlling [99] of toxoplasmosis involve transmission control, it depends on the host species on which we were focused but they are always based on reducing the exposure to T. gondii oocysts in the environment and avoid the ingestion of meat potentially contaminated with tissue cysts [100-103].
The implementation of farm biosecurity protocols, hygienic measures and management [104-106] practices should be adopted in all farms, mainly for reducing the level of environmental contamination with T. gondii oocysts [107-108] via cat faeces or limiting the access of felids to abortion-derived tissues. Basic measurements are: avoiding cat access to farm areas, especially to those housing pregnant ruminants, troughs food warehouses and water supplies; promptly removing any abortion-derived tissue as well as appropriately disposing of animal carcasses [109] and establishing rodent control. In humans, consumption habits, preventing to eat raw or undercooked [110] meat in which possible tissue cysts remain viable or washing vegetables and fruit before consumption, as well as to the way we handle domestic cats [111-116].
Conclusion and Recommendations
The review pact on Toxoplasma gondii is an infectious disease that is caused by an opportunistic of apicomplexan parasite globally distributed with a heteroxenous life cycle that virtually comprises all homoeothermic animals including humans, as intermediate hosts and felids as definitive hosts. The zoonotic, abortifacient and foodborne nature of the parasite makes toxoplasmosis a relevant public, animal health and the socio-economic impact concern of worldwide. Because highly complex process the versatility of T. gondii and its epidemiology, it is not possible to direct approaches for prevention and control of Toxoplasma gondii that are applied effectively worldwide.
Based on the conclusion the following recommendations are forwarded.
- Creating public awareness about toxoplasmosis should be promoted and Team work between veterinarian and human health physician is should be encouraged to increase the public awareness about toxoplasmosis, to break its associated risk factors and transmission pathways, via health extension service in rural and urban communities.
- Strategies to prevent exposure of T. gondii should focus on improvement of management farm sanitation, feed hygiene, rodent control and prevention of access of cats to farms.
- Health education should be given with special consideration to pregnant women and immunosuppresed individuals regarding minimizing contact with cats, avoidance of consumption of raw meat, vegetables and milk and unboiled river water also recommended.
- Not to allow eating of raw animal products must cooked animal byproducts should be practiced.
- Proper hygienic practices should be exercised with the aim of reducing contamination of drinking waters and food by cat feces.
- Continuing education and refresh training should be given to health professionals to upgrade the knowledge and perception on congenital toxoplasmosis.
- Clinical cases are treated with antibiotics and Intensive supportive treatment necessary in animals with disseminated disease.
References
- Damen JG, Luka J, Biwan EI, Lugos M (2011) Prevalence of intestinal parasites among pupils in rural North Eastern, Nigeria. Niger Med J 52: 4-6.
- WHO (2004) Regional Office for Africa “Developing and Maintaining Food Safety 60 Control Systems for Africa Current Status and Prospects for Change”, Second FAO/WHO Global Forum of Food Safety Regulators, Bangkok, Thailand, pp. 12-14.
- Maruyama S, Boonmar S, Morita Y, Sakai T, Tanaka S, et al. (2000) Seroprevalence of Bartonella henselae and Toxoplasma gondiiamong healthy individuals in Thailand. J Vet Med Sci 62: 635-637.
- Sougata G (2018) Paniker's Textbook of Medical Parasitology. Kol kata, West Bengal, India. Eighth Edition: Jaypee Brothers Medical Publishers: Pl Ltd. pp.90-120
- Grigg ME, Boothroyd JC (2001) Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondiiby PCR-restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol 39: 398-400.
- Khan A, Taylor S, Ajioka JW, Rosenthal BM, Sibley LD (2009) Selection at a single locus leads to widespread expansion of Toxoplasma gondii lineages that are virulent in mice. PLoS Genet5: 1000404.
- Su C, Howe DK, Dubey JP, Ajioka JW, Sibley LD (2002) Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proc Natl Acad Sci U S A99: 10753-10758.
- Aspinall TV, Joynson DH, Guy E, Hyde JE, Sims PF (2002) The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Infect Dis 185: 1637-1643.
- Carme B, Demar M, Ajzenberg D, Darde ML (2009) Severe acquired toxoplasmosiscaused by wild cycle of Toxoplasma gondii, French Guiana. Emerg Infect Dis 15: 656-658.
- Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30: 1217-1258.
- Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O (2011) Intracellular transport of Toxoplasma gondiithrough the blood-brain barrier. J Neuroimmunol 232: 119-130.
- Lukesova D, Literák I (1998) Shedding of Toxoplasma gondiioocysts by Felidae in zoos in the Czech Republic. Vet Parasitol 74: 1-7
- Stelzer S, Basso W, Benavides-Silván J, Ortega-Mora LM, Maksimov P, et al. (2019) Toxoplasma gondiiinfection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol 15: e00037.
- Pappas G, Roussos N, Falagas ME (2009) Toxoplasmosissnapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39:1385-1394.
- Teutsch SM, Juranek DD, Sulzer A, Dubey J, Sikes RK (2000) Epidemic toxoplasmosisassociated with infected cats. N Engl J Med 300: 695-699.
- Masur H, Jones TC, Lempert JA, Cherubini TD (2000) Outbreak of toxoplasmosisin a family and documentation of acquired retinochoroiditis. Am J Med 64: 396-402.
- Louis MW, Kim K (2013) Toxoplasma gondii: the model apicomplexan Elsevier.The Journal of protozoology. 34: 217-226.
- Flegr J, Preiss M, Klose J, Havlicek J, Vitáková M, et al. (2003) Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondiiDopamine, a missing link between schizophrenia and toxoplasmosis? Biol Psychol 63: 253-268.
- Gamble HR, Dubey JP, Lambillote DN (2005) Comparision of a commercial ELISA with the modified agglutination test for detection of Toxoplasmainfections in the domestic pig. Vet Parasitol 128: 177-181.
- CDC (2000) CDC recommendations regarding selected conditions effecting women's health. Morbidity and Mortality Weekly Report. 49: 57-75
- Swapna LS, Parkinson J (2017) Genomics of apicomplexanparasites. Crit Rev Biochem Mol Biol 52: 254-273.
- Nicolle C, Manceaux LH (1908) Surune infection a corps de Leishman (ou organismes voisins) du gondi. C R Seances Acad Sci147: 763-766.
- Wolf A, Cowen D (1937) Toxoplasmicencephalomyelitis: IV. Experimental transmission of the infection to animals from a human infant. J Exp. Med. 71:187–214.
- Wolf A, Cowen D, Paige B (1939) Human toxoplasmosis: occurrence in infants as an encephalomyelitis verification by transmission to animals. Science89: 226-227.
- Sabin AB, Olitsky PK (1937) Toxoplasmaand obligate intracellular paarsitism. Science 85: 336-338.
- Dubey JP, Jones JL (2008) Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol38: 1257-1278.
- Isaac-Renton J (1998) Detection of Toxoplasma gondiioocysts in drinking water. Applied and environmental microbiology 64: 2278-2280.
- Pena HFJ, Evangelista CM, Casagrande RA, Biezus G, Wisser CS, et al. (2017) Fatal toxoplasmosisin an immunosuppressed domestic cat from Brazil caused by Toxoplasma gondii clonal type I. Rev Bras Parasitol Vet 26: 177-184.
- Stajner T, Vasiljevic Z, Vujic D, Markovic M, Ristic G, et al. (2013) Atypical strain of Toxoplasma gondiicausing fatal reactivation after hematopoietic stem cell transplantion in a patient with an underlying immunological deficiency. J Clin Microbiol 51: 2686-2690.
- Delhaes L, Mraz JC, Fréalle E, Durand-Joly I, Magro L, et al. (2010) Severe pulmonary toxoplasmosisafter allo-SCT in two patients: from Toxoplasma genotyping to clinical management. Bone Marrow Transplant 45: 580-583.
- Ferguson DJP, Dubremetz JF (2014) The ultrastructure of Toxoplasma gondii, in: Toxoplasma Gondii(Second Edition). Academic Press, Boston, 19-59.
- Martorelli D, Genova B, Wilson SK, Dubey JP, Knoll LJ (2019) Intestinal delta-6-desaturase activity determines host range for Toxoplasmasexual reproduction. PLoS Biol 17: e3000364.
- Jones JL, Dubey JP (2010) Waterborne toxoplasmosis--recent developments. Exp Parasitol124: 10-25.
- Getachew A, Tilahun A, Aylate A, Tesfaye W (2016) Sero-Prevalence of ToxoplasmaGondii Infection and Associated Risk Factors in Animals Presented to Sholla and Akaki-Kality Veterinary Clinics, Addis Ababa. Global Journal of Medical Research: G Veterinary Science and Veterinary Medicine 16: 2249-4618.
- Acha PN, Szyfres B [PAHO]). (2003) Zoonoses and communicable diseases common to man and animals. Volume 3. Parasitoses. 3rd ed. Washington DC: PAHO; Scientific and Technical Publication 580. Toxoplasmosis; p. 76-86
- Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, et al. (2002) Genotype of 86 Toxoplasma gondiiisolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis 186: 684- 689.
- Innes EA (2010) A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 57: 1-7.
- Ramakrishnan C, Maier S, Walker RA, Rehrauer H, Joekel DE, et al. (2019) An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondiiby cats. Sci Rep 9: 1474.
- Robert-Gangneux F, Dardé ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev25: 264-296.
- Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, et al. (2000) Sources of toxoplasmainfection in pregnant women: European multicentre case-control study. Br Med J 321: 142-147.
- Schlüter D, Barragan A (2019) Advances and challenges in understanding cerebral toxoplasmosis. Front Immunol10: 242.
- Gavinet MF, Robert F, Delouvrier E, Hennequin C, Maurin JR, Tourte-Schaefer, C. and Dupouy-Camet, J (1997) Congenital toxoplasmosisdue to maternal reinfection during pregnancy. Journal of Clinical Microbiology 35: 1276-1277.
- Vogel N, Kirisits M, Michael E, Bach H, Hostetter M, et al. (1996) Congenital ToxoplasmosisTransmitted from an Immunologically Competent Mother Infected Before Conception. Clin Infect Dis 23: 1055-1060.
- Sah RP, Dey AR, Rahman AA, Alam MZ, Talukder MH (2019) Molecular detection of Toxoplasma gondiifrom aborted fetuses of sheep, goats and cattle in Bangladesh. Vet Parasitol Reg Stud Reports 18:100-347.
- Sánchez-Sánchez R, Vázquez P, Ferre I, Ortega-Mora LM (2018) Treatment of toxoplasmosisand neosporosis in farm ruminants: state of knowledge and future trends. Curr Top Med Chem 18: 1304-1323.
- Shibre T, Alem A, Abdulahi A, Araya M, Beyero T, et al. (2010) Trimethoprim as adjuvant treatment in schizophrenia: a double-blind, randomized, placebo controlled clinical trial. Schizophr Bull 36: 846-851.
- Shimelis T, Tebeje M, Tadesse E, Tegbaru B, Terefe A (2009) Seroprevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia: a comparative cross-sectional study. BMC Res Notes 50: 213.
- Nayeri T, Sarvi S, Moosazadeh M, Amouei A, Hosseininejad Z, Daryani A (2021) Anti-Toxoplasma gondiiantibodies in European citizens within the last 20 years: A systematic review and meta-analysis. J.Vet Res 56: 9-22.
- Frenkel JK, Hassanein KM, Hassanein RS, Brown E, Thulliez P, et al. (2002) Transmission of Toxoplasma gondiiin Panama City, Panama: a five-year prospective cohort study of children, cats, rodents, birds, and soil. Am J Trop Med Hyg 53: 458-468.
- Bartoszcze M, Krupa K, Roszkowski J (2007) ELISA for assessing Toxoplasmagondii antibodies in pigs. J Vet Med 38: 263-264.
- Morrissette NS, Sibley LD (2002) Cytoskeleton of Apicomplexan Parasites. Microbiology and Molecular Biology. Reviews 66: 21-38.
- Teshale S, Dumètre A, Dardé ML, Merga B, Dorchies P (2007) Serological survey of toxoplasmosis in Ethiopia: prevalence and risk factors. J Parasite 14: 155-159.
- Jilo K, Tegegne D, Kasim S, Dabasa G, Zewdei W (2021) Seroprevalence and Public Health Significance of Toxoplasmosisin Small Ruminants of Pastoral Community in Yabello District, Borana Zone, Southern Ethiopia. J. Veterinary Medicine International 11: 102-120.
- Negash T, Tilahun G, Patton S, Prevot F, Dorchies PH (2004) Serological survey of toxoplasmosis in sheep and goats in Nazareth, Ethiopia. Revue Med Vet 155: 486-487.
- Bekele T, Kasali, OB (1989) Toxoplasmosisin sheep, goats and cattle in central Ethiopia. Veterinary Research Communications 13: 371-375.
- Yimer E, Abebe P, Kassahun J, Woldemichael T, Bekele A, et al. (2008) Seroprevalence of Toxoplasmosis Addis Ababa, Ethiopia. Ethio Vet J9: 109-122.
- Hill D, Dubey JP (2002) Toxoplasma gondii: Transmission, diagnosis and prevention. Clinical Microbiology and Infection8: 634-640.
- Yibeltal M (2005) Study of toxoplasmosisin small ruminants and Humans (HIV/AIDS Patients) in selected district of South Wollo, Ethiopia. MSc Thesis, Faculty of veterinary Medicine. Addis Abeba University, Bishoftu, Ethiopia 1-2.
- Goldstein EJ, Montoya JG, Remington JS (2008) Management of Toxoplasmagondii infection during pregnancy. Clinical Infectious Diseases 47: 554-566.
- Rashidi S, Sadraei J, Jafari-Modrek M (2013) Comparison of Giemsa staining, intraperitoneal injection and oral administration methods in rat brain infected with Toxoplasma gondii. ZahedanJ Res Med Sci 16: 45-49.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2006) Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. Sounders, London: 1518-1522.
- Belluco S, Mancin M, Conficoni D, Simonato G, Pietrobelli M, et al. (2016) Investigating the determinants of Toxoplasma gondiiprevalence in meat: A systematic review and meta-regression. PLoS ONE 11: 15-38.
- Opsteegh M, Kortbeek TM, Havelaarand AH, Van Der Giessen JW (2014) Interventionstrategies to reduce human Toxoplasma gondiidisease burden. Clinical Infectious Diseases 7: 21.
- Opsteegh M, Maas M, Schares G, Giessen J (2016) Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondiiin meat (GP/EFSA/BIOHAZ/2013/01) an extensive literature review. Final report. EFSA Supporting Publications 13: 996.
- Lawley R, Curtis L, Davis J (2006) The Food Safety Hazard Guide book. RSC Publishing.
- Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T (2001) Obsessivecompulsive disorder and acquired toxoplasmosisin two children. European Child & Adolescent Psychiatry 10: 200-204.
- Dechassa T, Amin k, Mukarim A, Moti Y (2016) Seroepidemiology and associated risk factors of Toxoplasma gondii in sheep and goats in Southwestern Ethiopia. BMC Veterinary Research12: 280.
- Nayeri T, Sarvi S, Moosazadeh M, Daryani A (2021) Global prevalence of Toxoplasma gondii infection in the aborted fetuses and ruminants that had an abortion: A systematic review and meta-analysis. Vet Parasitol 290: 109-370.
- Blader IJ, Saeij JP (2009) Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. Apmis 117: 458-76.
- Castaño P, Fuertes M, Ferre I, Fernández M, Ferreras MC, et al. (2014) Placental thrombosis in acute phase abortions during experimental Toxoplasma gondii infection in sheep. Vet Res 45: 9.
- Rico-Torres, Vargas-Villavicencio CP, Correa, JA (2016) Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review. Eur J Clin Microbiol Infect Dis 35: 1079-1088.
- Mukhopadhyay D, Arranz-Solís D, Saeij JPJ (2020) Influence of the host and parasite strain on the immune response during Toxoplasma Front Cell Infect Microbiol. 10: 580425.
- Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J, Sher A (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe. 15: 132-138.
- Collazos J (2003) Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 17: 869-887.
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, et al. (2007) Fatal Outbreak of human toxoplasmosis along the Maroni River Epidemiol Parasitol. Infect Dis 45: 88-95.
- Liu Q, Wang ZD, Huang SY, Zhu XQ (2015) Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors 8: 292.
- Sroka J, Wojcik-Fatla A, Szymanska J, Dutkiewicz J, Zajac V et al. (2010) The occurrence of Toxoplasma gondii infection in people and animals from rural environment of Lublin region - estimate of potential role of water as a source of infection. Ann Agric Environ Med 17: 125-132.
- Dubey JP (2010) Toxoplasmosis of Animals and Humans 2nd edition CRC Press Boca Raton, Florida, USA 1-313.
- Benavides J, Fernández M, Castaño P, Ferreras MC, Ortega Mora L et al. (2017) Ovine toxoplasmosis: a new look at its pathogenesis. J Comp Pathol 157: 34-38.
- Grigg ME, Ganatra J, Boothroyd JC, Margolis TP (2001) Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis 184: 633-639.
- Cold CJ, Sell TL, Reed KD (2005) Diagnosis Disseminated Toxoplasmosis. Clinical Medicine & Research 3: 186-186.
- Montoya JG (2002) Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J of Infectious Diseases 185: 73-82.
- Buxton D (2000) Toxoplasmosis and neosporosis. In: Diseases of Sheep, Martin W.B. and Aitken Blackwell Science Oxford UK 18: 86-94.
- Paul M (2008) Potential risk factors for Toxoplasma gondii infection in cases with recently acquired toxoplasmosis. Przegl Epidemiol 52: 447-454.
- Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Yang YR et al. (2020) All about toxoplasmosis in cats: the last decade. Vet Parasitol 283: 109145.
- Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, et al. (2009) Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. The Journal of Infectious Diseases 199: 1155-1167.
- Aleme H, Tilahun G, Fekade D, Berhe N, Medhin G (2012) Sereoprevalence of Immunoglobulin-G and of Immunoglobulin-M anti-Toxoplasma gondii antibodies in Human Immunodeficiency Virus Infection/Acquired.
- Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T (2001) Obsessivecompulsive disorder and acquired toxoplasmosisin two children. European Child & Adolescent Psychiatry 10: 200-204.
- Dechassa T, Amin k, Mukarim A, Moti Y (2016) Seroepidemiology and associated risk factors of Toxoplasma gondii in sheep and goats in Southwestern Ethiopia. BMC Veterinary Research12: 280.
- Demissie T, Tilahun G (2002) Study on toxoplasmosis in sheep and goats in Debre Birhan and surrounding areas in Ethiopia. Bull Anim Hlth Prod Afr50: 138-147.
- Dubey JP, Lindsay D, Speer C (1998) Structures of Toxoplasmagondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical microbiology reviews 11: 267-299.
- Dubey JP (2001) Oocysts shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondiito cats and mice. J Parasitol 87: 215-219.
- Dubey JP (2004) Toxoplasmosis: A waterborne zoonosis. Vet Parasitol126: 57-72.
- Dubey JP (2006) Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondiifor intermediate (mice) and definitive (cats) hosts. Vet Parasitol 140: 69-75.
- Dubey JP (2009) Toxoplasmosisin pigs. The last 20 years. Vet Parasitol 164: 89-103.
- Dubey JP (2010) Toxoplasmosisof Animals and Humans 2nd edition. CRC Press; Boca Raton, Florida, U.S.A 1-313.
- Dubey JP, Darrington C, Tiao N, Ferreira LR, Choudhary S, et al. (2013) Isolation of viable Toxoplasma gondii from tissues and feces of cats from Addis Ababa, Ethiopia. J Parasitol99: 56-58.
- Dubey JP, Kotula AW, Sharar A, Andrews CD, Lindsay DS (1990) Effect of high temperature on infectivity of Toxoplasma gondiitissue cysts in pork. J Parasitol 76: 201-204.
- Dubey JP, Zhu XQ, Sundar N, Zhang H, Kwok OCH, et al. (2007) Genetic and biologic characterization of Toxoplasma gondiiisolates of cats from China. Vet Parasitol 145: 352-356.
- Gebremedhin EZ, Yunus HA, Tesfamaryam G, Tessema TS, Dawo F et al. (2014) First report of Toxoplasma gondii in camels (Camelus dromedarius) from Ethiopia: seroepidemiology and bioassay. BMC Vet Res12: 140-222.
- Hassan MA, Olijnik AA, Frickel EM, Saeij JP (2019) Clonal and atypical Toxoplasmastrain differences in virulence vary with mouse sub-species. Int J Parasitol 49: 63-70.
- Howe DK, Honoré S, Derouin F, Sibley LD (1997) Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. Journal of Clinical Microbiology 35: 1411-1414.
- Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet363: 9425
- Olivier A, Herbert B, Sava B, Pierre C, John D, et al. (2007) Surveillance and monitoring of Toxoplasmain humans, food and animals. EFSA J 583: 1-64.
- Petersen E, Eaton RB (2009) Control of congenital infection with Toxoplasma gondiiby neonatal screening based on detection of specific immunoglobulin M antibodies eluted from phenylketonuria filter-paper blood-spot samples. Acta Paediatr Suppl 88: 36-39.
- Reischl U, Bretagne S, Krüger D, Ernault P Costa JM (2003) Comparison of two DNA targets for the diagnosis of Toxoplasmosisby real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infectious Diseases 3: 7.
- Smith DD (1988) The Sarcocystidae: Sarcocystis, Frenkelia, Toxoplasma, Besnoitia, Hammondia, and Cystoisospora. J Protozool28: 262-266.
- Tiao N, Darrington C, Molla B, Saville WJ, Tilahun G, et al. (2013) An investigation into the sero prevalence of Toxoplasma gondii, Bartonella spp feline immunodeficiency virus (FIV) and feline leukemia virus (FelV) cats from Addis Ababa. Epidemiol Infect 141: 1029-1033.
- Tilahun B, Tolossa YH, Tilahun G, Ashenafi H, Shimelis S (2018) Seroprevalence and Risk Factors of Toxoplasma gondiiInfection among Domestic Ruminants in East Hararghe Zone of Oromia Region, Ethiopia. Veterinary medicine international
- Tilahun G, Tiao N, Ferreira LR, Choudhary S, Oliveira S (2013) Seroprevalence of Toxoplasma gondii from free-range chicken (Gallus domesticus) from Addis Ababa, Ethiopia. Parasitol 12: 25.1
- Teshale S, Dumètre A, Dardé ML, Merga B, Dorchies P (2007) Serological survey of caprine toxoplasmosisin Ethiopia: prevalence and risk factors. Parasite 14: 155–159.
- Weiss LM, Dubey JP (2009) Toxoplasmosis: A history of clinical observations. J. Parasitol 39: 895-90.
- Zemene E, Yewhalaw D, Abera S, Belay T, Samuel A (2012) Seroprevalence of Toxoplasma gondii and associated risk factors among pregnant women in Jimma town, Southwestern Ethiopia. BMC Infect Dis 14: 12-37.
- Zewdu E, Agonafir A, Tessema TS, Tilahun G, Medhin G, et al. (2013) Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res Vet Sci. 94:43-8.
- Paul M (2008) Potential risk factors for Toxoplasma gondii infection in cases with recently acquired toxoplasmosis. Przegl Epidemiol 52: 447-454.
- Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Yang YR et al. (2020) All about toxoplasmosis in cats: the last decade. Vet Parasitol 283: 109145.
Citation: Woldesenbet YB, Harito JB, (2023) Epidemiology and Public Health Importance of Toxoplasma Gondii in Ethiopia: An Overview J Anim Res Vet Sci 7: 0.44
Copyright: © 2023 Yehualashet Behailu Woldesenbet, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

