
Ewing Sarcoma/Primitive Neuroectodermal Tumors in the Mediastinum: A Case Report and Review of Literature
*Corresponding Author(s):
Qiang DuDepartment Of Respiratory And Critical Care Medicine, The Second Affiliated Hospital Of Nanjing Medical University, Nanjing, China
Tel:+86 02558509837,
Email:jingshuyue@163.com
Jingning Liu
Department Of Respiratory And Critical Care Medicine, The Second Affiliated Hospital Of Nanjing Medical University, Nanjing, China
Email:jingningliu1221@163.com
Abstract
Ewing Sarcoma/Primitive Neuroectodermal Tumors (ES/PNETs) are highly malignant neoplasms originating from primitive neural tube embryonic cells, and grouped in the Ewing family of tumors. These malignancies are relatively uncommon, most frequently arising from bones or soft tissues and rarely reported in the mediastinum. The present study describes a case of a 22-year-old woman with unresectable ES/PENT in the middle mediastinum. The patient died the next day after bronchoscopy. A review of the literature showed reports of 9 patients with mediastinal PENTs in the past 20 years. The therapy of choice for ES/PENT in mediastinum consists of combinations of surgical excision with neoadjuvant or adjuvant chemotherapy and radiation to improve event-free survival rates.
Keywords
Clinical characteristics; Diagnosis; Mediastinum; Primitive neuroectodermal tumors
INTRODUCTION
Ewing Sarcoma/Primitive Neuroectodermal Tumors (ES/PNETs) are primary malignant neoplasms of bone and soft tissues, grouped in the family of small round tumor cells [1]. These uncommon tumors primarily affect children and young adults, but may occur in other age groups [2,3]. Extra-skeletal ES/PENTs has been described in soft tissues at any locations, such as kidney, pancreas, and myocardium, but primary mediastinal location of ES/PENT is extremely rare [2,4]. Diagnosis of the tumor is confirmed using immunohistochemical studies and gene detection of t(11;22) translocation by fluorescent in situ hybridization [4]. ES/PENTs can be treated with various combinations of surgical resection, neoadjuvant and adjuvant chemotherapy and irradiation [5].
CASE DESCRIPTION
We describe the case of a 22-year-old nonsmoking woman who first presented to the Nanjing Chest Hospital with fever, cough and blood in phlegm, chest tightness and orthopnea. The patient had no significant medical and family history of tumors. A routine physical examination was not conducted on the patient because of orthopnea. No abnormality was found in blood routine and biochemistry examination. However, serum D-dimer level [(15.83ug/ml) (normal range, <1/ µg/ml)] and the serum tumor marker level of Carbohydrate Antigen (CA) 125[455.3U/ml (normal range, <35U/ml)], were higher than normal levels.
Echocardiogram and chest Computed Tomography (CT) (Figure 1) showed pericardial effusion. Pericardiocentesis was performed, and the examination of pericardial effusion suggested the possibility of malignancy. Chest CT with contrast demonstrated a large mass in the middle mediastinum with pericardial effusion, left pleural effusion and initial compression of right pulmonary artery (Figure 1). Closed thoracic drainage with thin tube was performed in the left thoracic cavity. The pleural effusion was bloody and proved to be exudative. The tumor marker examination of pleural effusion showed CA125 934U/ml (normal range, <35U/ml) and Cytokeratin Fragment (CYFRA) 211 178.20ng/ml (normal range, <3.3ng/ml). Unfortunately, tumor cells were not found in pleural effusion.
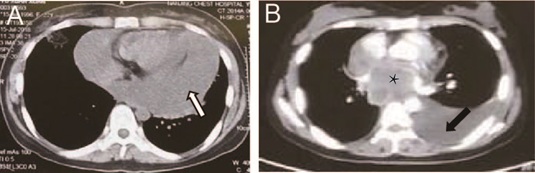 Figure 1: Representative images fron the chest Computed Tomography (CT) scan and heart contrast-enhanced CT scan of the 22-year-old patient. (A) Chest CT scan with mediastinal window image showed pericardial effusion (white arrow). (B) Heart contrast-enhanced CT scan showed left pleural effusion (black arrow) and one mass in middle mediastinum (black asterisk).
Figure 1: Representative images fron the chest Computed Tomography (CT) scan and heart contrast-enhanced CT scan of the 22-year-old patient. (A) Chest CT scan with mediastinal window image showed pericardial effusion (white arrow). (B) Heart contrast-enhanced CT scan showed left pleural effusion (black arrow) and one mass in middle mediastinum (black asterisk).
The patient was admitted to our hospital for further treatment. A biopsy of the mediastinal mass was performed (endobronchial ultrasound guided transbronchial needle aspiration, EBUS-TBNA). Because the patient had severe orthopnea?the fiberoptic bronchoscopy examination was performed in a sitting position (Figure 2). Histopathologic examination of the specimen indicated malignant, small, round-cell tumor. The immunohistochemical results demonstrated that some of the cells were positively stained for CD99, Ki-67 (Figure 3) and negatively stained for CD5, CD125, Cg-A and PLAP. Based on these findings, an ES/PNET was diagnosed. This patient died the next day after the EBUS-TBNA examination.
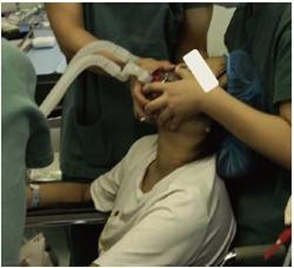 Figure 2: The fiberoptic bronchoscopy examination was performed by anesthesia in a sitting position because the patient had orthopnea.
Figure 2: The fiberoptic bronchoscopy examination was performed by anesthesia in a sitting position because the patient had orthopnea.
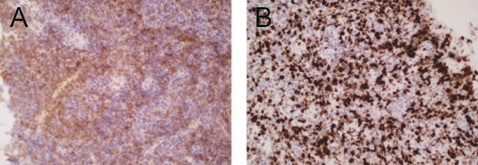 Figure 3: Immunohistochemical analysis of tumor tissue. (A) Immunohistochemical staining of CD99. CD99 are shown as brown area counterstained with hematoxylin. (B) Immunohistochemical staining of Ki67. Ki67 are shown as brownish black dots counterstained with hematoxylin. Original magnification, ×400.
Figure 3: Immunohistochemical analysis of tumor tissue. (A) Immunohistochemical staining of CD99. CD99 are shown as brown area counterstained with hematoxylin. (B) Immunohistochemical staining of Ki67. Ki67 are shown as brownish black dots counterstained with hematoxylin. Original magnification, ×400.
DISCUSSION
ES/PNET is a rare and highly malignant soft-tissue tumor. Peripheral PNETs are rare and high-grade malignant neoplasms, originating from primitive neural tube embryonic cells, and grouped in the Ewing family of tumors [2,6]. These malignancies are characterized by a chromosome 22 rearrangement, most frequently arising from bones or soft tissues, but they have been rarely reported in other sites, such as ovary, uterus, kidney, lung, and pancreas [7]. About 80% of patients suffer these highly malignant tumors in their first 2 decades of life [2,5].
Peripheral PNETs in the thorax most commonly originate in the lung [5]. Among the thoracic ES/PENTs, primary mediastinal PENTs are very rare. Fewer than 10 primary PNETs in the mediastinum have been reported in the past 20 years (1998-2019). Review of these literatures showed a female preponderance (F:M =2:1), with a mean age of 41.3 (3.5-75) years [4,8-15]. Our case was younger in age than most of previously reported patients in the literature. The cases of reported primary PNET in mediastinum are summarized in table 1.
|
Case |
year |
Sex |
Age |
Tumor location |
Treatment |
Follow-up |
Reference |
|
1 |
2016 |
M |
42 |
Anterior and middle M |
Resection only |
A&W at 4 months |
8 |
|
2 |
2015 |
F |
51 |
Posterior M |
CRT |
NK |
9 |
|
3 |
2013 |
F |
66 |
Anterior M |
CRT |
NK |
4 |
|
4 |
2013 |
M |
75 |
Anterior M |
CT only |
A&W at 4 months |
10 |
|
5 |
2013 |
F |
64 |
Anterior M |
Resection /adjuvant CT |
A&W at 120 months |
11 |
|
6 |
2013 |
M |
3.5 |
Anterior M |
Resection/adjuvant CT |
A&W at 2months |
12 |
|
7 |
2010 |
F |
15 |
Posterior M |
Resection only |
A&W at 12months |
13 |
|
8 |
2008 |
F |
24 |
Posterior M |
Resection/adjuvant CT |
NK |
14 |
|
9 |
1998 |
F |
32 |
Posterior M |
Resection/adjuvant CT |
DOD at 22months |
15 |
Table 1: Patients with primary ES/PNET in mediastinum in the literature.
M: Male, F: Female, M: Mediastinum, CT: Chemotherapy, CRT: Chemoradiation, NK: Not Knoun, A&W: Alive and Well, DOD: Dead of Disease.
Cough, fever, dyspnea, hemoptysis and chest pain are presenting symptoms of thoracic PNETs. The imaging performance of tumors lacks specificity, and it is difficult to diagnosis before operation and easy to be misdiagnosed [16]. On chest CT scan, mediastinal PENT usually manifests as a large soft tissue in chest with smooth edges, uneven density and (or) liquefaction. Enhanced CT scan shows the tumor as a unilateral heterogeneously enhanced mass, often infiltrating adjacent structure such as vessels, pleura or pericardium, to induce pleural effusion or pericardial effusion. However, calcification of the mass and lymph mode metastasis are rarely seen [2,8,17].
In our case, cough and progressive dyspnea were the presenting symptoms. The mediastinal tumor revealed relatively typical CT features. Most of the large mass was located in middle mediastinum, with heterogeneously enhanced large mass infiltrating left pleura, pericardium and right pulmonary artery, with left pleural effusion and pericardial effusion. No calcification and lymph node metastasis was identified at the time of initial diagnosis.
The differential diagnosis of thoracic PNETs includes small cell lung carcinoma, malignant lymphoma, classical neuroblastoma, and granulocytic sarcoma, etc. The diagnosis was confirmed by performing histochemical and immunohistochemical studies. The classic histologic morphological features of PENT is small round blue cells with uniform size, hyperchromatic nuclei and scant eosinophilic cytoplasm [2,3,18]. Wright-type rosettes might be identified in PNET biopsy [17]. These histologic patterns are not specific morphologic features, and immunohistochemical staining is helpful to differentiate PNET from other small round blue cell tumors [2,14,18]. CD99 is a sensitive and diagnostic marker of PNET, and its positive rate is up to 80%-95% [2,3,18]. Positive findings of other markers, including NSE, vimentin, also can be helpful in diagnosis of PENT. Identification of t(11;22)(q24;q12) chromosomal translocation (EWS-FLI1 gene fusion) by molecular genetic techniques is also highly specific for ES/PNET [14,19].
The standard treatment for PNET is the combination of surgical resection, neoadjuvant and neoadjuvant chemotherapy and radiotherapy [11]. The chemotherapy is based on anthracyclines and alkylating agents combined with other chemotherapy drugs [8]. There are no related reports on treatment with target drugs until now. Most important influencing factors for prognosis are tumor size, the accompanying complication of local infiltration and compression, and presence of distant metastasis [3,14]. The main cause of PNET treatment failure is local recurrence and distant metastasis. However, multimodality treatment programs could achieve event-free survival rates of 60% [4]. In our case, chemotherapy and radiation therapy were not performed due to patient’s death on the day after EBUS-TBNA examination, and the clinical course of the patient well represented highly malignant characteristics and behavior of the PENT as previously reported [2,6].
Although patients with ES/PNET arising from mediastinum are extremely rare, they should be considered in differential diagnosis of patients with mediastinal mass, particularly in the younger patients. Understanding the rare disease can help clinicians to approach efficient diagnosis and better treatment for this highly malignant tumor.
ACKNOWLEDGEMENT
This research was supported by the The Six Talent Peak Projects of Jiangsu Province (No: 2015100110268).
CONFLICT OF INTEREST
None
DISCLOSURES
None
REFERENCES
- Andrei M, Cramer SF, Kramer ZB, Zeidan A, Faltas B (2013) Adult primary pulmonary primitive neuroectodermal tumor molecular features and translational opportunities. Cancer Biol Ther 14: 75-80.
- Zhang WD, Zhao LL, Huang XB, Cai PQ, Xu GX (2010) Computed tomography imaging of anterior and middle mediastinal Ewing sarcoma/primitive neuroectodermal tumors. J Thorac Imaging 25: 168-172.
- El Weshi A, Allam A, Ajarim D, Al Dayel F, Pant R, et al. (2010) Extraskeletal Ewing’s sarcoma family of tumours in adults: analysis of 57 patients from a single institution. Clin Oncol (R Coll Radiol) 22: 374-381.
- Reali A, Mortellaro G, Allis S, Trevisiol E, Anglesio SM, et al. (2013) A case of primary mediastinal Ewing's sarcoma / primitive neuroectodermal tumor presenting with initial compression of superior vena cava. Ann Thorac Med 8: 121-123.
- Weissferdt A, Moran AC (2012) Primary Pulmonary Primitive Neuroectodermal Tumor (PNET): A clinicopathological and immunohistochemical study of six cases. Lung 190: 677-683.
- Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, et al. (2011) A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol 197: 1015-1022.
- Takeuchi T, Iwasaki H, Ohjimi H, Kaneko Y, Ishiguro M, et al. (1996) Renal primitive neuroectodermal tumor: Animmunohistochemical and cytogenetic Analysis. Pathology Internationa l 46: 292-297.
- Bae SH, Hwang JH, Da Nam B, Kim HJ, Kim KU, et al. (2016) Multiple ewing sarcoma/primitive neuroectodermal tumors in the mediastinum: A case report and literature review. Medicine (Baltimore) 95: 2725.
- Liu M, Liu B, Dong L, Han T, Zhang L (2015) Extraskeletal Ewing's sarcoma/primitive neuroectodermal tumor of the mediastinum: Significant response to chemoradiotherapy. Oncol Lett 9: 626-628.
- Kalkan KE, Bilici A, Selcukbiricik F, Unver N, Yuksel M (2013) Thoracic primitive neuroectodermal tumor: An unusual case and literature review. Case Rep Pulmonol 2013: 326871.
- Sirivella S, Gielchinsky I (2013) Treatment outcomes in 23 thoracic primitive neuroectodermal tumours: a retrospective study. Interact Cardiovasc Thorac Surg 17: 273-279.
- Wu Z, Wan H, Shi M, Gao W, Wang Z, et al. (2013) Giant primitive nuroectodermal tumor of mediastinum: A case report with literature review. Zhongguo Fei Ai Za Zhi 16: 273-276.
- Chang SI, Tsai MC, Tsai MD (2010) An unusual primitive neuroectodermal tumor in the thoracic epidural space. J Clin Neurosci 17: 261-263.
- Manduch M, Dexter DF, Ellis PM, Reid K, Isotalo PA (2008) Extraskeletal Ewing's sarcoma/primitive neuroectodermal tumor of the posterior mediastinum with t(11;22)(q24;q12). Tumori 94: 888-891.
- Inaba H, Ohta S, Nishimura T, Takamochi K, Ishida I, et al. (1998) An operative case of primitive neuroectodermal tumor in the posterior mediastinum. Kyobu Geka 51: 250-253.
- Gupta P, Hari S, Thulkar S (2013) Imaging spectrum of peripheral primitive neuroendocrine tumours. Singapore Med J 54: 463-469.
- Dick EA, McHugh K, Kimber C, Michalski A (2001) Imaging of non-central nervous system primitive neuroectodermal tumours: diagnostic features and correlation with outcome. Clin Radiol 56: 206-215.
- Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, et al. (2005) Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol 29: 1025-1033.
- Noguera R, Pellin A, Navarro S, Carda C, Llombart-Bosch A (2001) Translocation (10;11;12)(p14;q24;q12) characterized by fluorescence in situ hybridization in a case of Ewing's tumor. Diagn Mol Pathol 10: 2-8.
Citation: Han B, Zhu C, Weng X, Du Q, Liu J (2020) Ewing Sarcoma/Primitive Neuroectodermal Tumors in the Mediastinum: A Case Report and Review of Literature. J Pulm Med Respir Res 6: 040.
Copyright: © 2020 Bo Han, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

